Anatomical basis of pedicles in breast reduction
Introduction
The breast is shaped like a semi-sphere that rests on the thorax on its flat side, presenting at the center of its convex portion is the papillar nipple. Its shape offers countless variables; its anteroposterior diameter can lengthen (pear-shaped or conical breast) or shrink (flattened or discoid breast). In fat and multiparous women, instead of ending in a point, the breast retains an almost unvarying diameter (cylindrical breast). In other circumstances, it has a narrow diameter at its attachment to the chest (pedicle breast) (1-3).
The breast is approximately 8 to 10 millimeters in diameter at birth and remains in rudimentary form until puberty. From puberty, breasts achieve a growth rate reaching an average of 10 to 11 centimeters high by 12 to 13 centimeters wide and 5 to 6 centimeters thick. The adult breast region extends to the ribcage in a number of constituent planes where, from surface to depth, we highlight:
Anatomical structures of the breast region
Skin envelope
Representing the anterior surface of the region, convex, fixed at the inframammary fold downward, and spreading onto the skin of the infraclavicular region upward, the skin envelope is comprised of uniform, smooth skin with variable color according to race and covered with fine hair.
The areola is a typically circular region ranging from 15 to 25 mm in diameter according to some authors and up to 50 mm for others, with an average of 40 mm. It is clearly distinguishable from the skin in the rest of the region because of greater pigmentation and has a number of elevations or eminences, on average 12–24, called Morgagni’s tubercles, irregularly distributed across its surface.
Morgagni’s tubercles correspond to sebaceous glands which, being widely distributed raise the integument. At the center of each of these glands is a fine hair. During pregnancy, these nodules increase in size to 2 to 5 mm and remain enlarged during the entire breast feeding period, termed Montgomery’s tubercles.
At the center of the areola is the nipple, a raised formation, papillar in shape, facing forward and out. It typically resembles a cylinder or rounded cone, although it can present various forms such as flattened, discoid, umbilicated, and pedicled. The nipple, although variable, has an average length of 10 to 12 mm with a width of 9 to 10 mm (3-5).
The skin covers the anterior surface of the mammary gland and continues on the periphery to join the skin of the chest. Structurally, this skin differs depending on the breast area it covers; and it is divided for the purpose of this analysis into three concentric areas:
Subcutaneous factor tissue
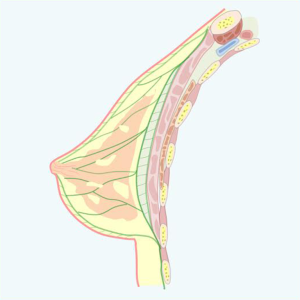
Thicker at the periphery and almost nonexistent on the areolar level, disappearing into the nipple (where the skin practically adheres to the gland), this layer continues with connective tracts that penetrate the gland and are called fibroglandular ridges of Duret (Figure 1).
Mammary fascias
The superficial fascia that lies beneath the subcutaneous tissue is divided into two sheets, superficial and deep, and the mammary gland is found between them. The mammary gland, whose embryological origin is ectodermal, being an epithelial derivative, is thus suspended between the two layers of the superficial fascia, a sagittal section of the mammary region, the superficial fascia (in green) is divided into two sheets covering the gland, constituting the pre- and retro-mammary fascias. Cleavage sites for modeling in which the vascular-nervous bundles are preserved. The surface or anterior mammary fascia divides subcutaneous fat into a surface sheet that adheres to the dermis and a deep sheet with a trabecular pattern directed toward the glandular parenchyma.
Mammary gland
The mammary gland is formed by multiple tubuloacinar pockets wherein each tubule consists of an inner layer of secretory cells and an outer layer of myoepithelial cells. In this manner, the breast tissue secretory gland is situated in a connective tissue stroma and fatty tissue, and all of these elements respond to hormonal and systemic influences. Each breast contains approximately 15 to 20 lobes, each consisting of lobules with excretory ducts that open into collecting ducts called galactophorous or milk ducts. These ducts widen distally at the level of the areola, forming the so-called lactiferous sinuses, which act as temporary reservoirs of milk during lactation.
The breast parenchyma considerably exceeds the limits of the region both macroscopically and microscopically, extending up to the clavicle and downward beyond the submammary fold. Inwardly, the midline can be reached and even passed almost without apparent continuity to the opposite side with the contralateral breast tissue. Outwardly, the limits of the lateral border of the pectoralis major can be exceeded. The tail or axillary extension of the breast can reach this topographic region, and breast tissue can even be found in fat or axillary pad tissue samples (6,7).
When speaking of breast fascias, pre- and retroglandular, we must mention Cooper’s ligament, one of the principal means of support in breast architecture. Cooper’s ligament, unlike most of the ligamentous structures of our economy, is not a well-defined fasciculus with proximal and distal insertions, paths, connections, and a defined anatomical entity, but 14 is rather represented by a series of trabeculae that cross the breast parenchyma and extend from the premammary fascia to the retromammary fascia (Figure 1).
Retromammary fat and fascia
Found behind the mammary gland, the retromammary fat, just like that situated in front of the gland, sends expansions to the glandular parenchyma. Behind this gland is the retromammary gland fascia from which the aforementioned Cooper’s ligament originates.
Retromammary areolar tissue
Deep mammary or retromammary fascia is separated from the fascia of the pectoralis major muscle by easily displaced areolar tissue, referred to as “loose connective tissue” or “areolar connective tissue” as stated (Figures 2,3).
Pectoralis major muscle
The mammary region extends in depth to the rib cage, which is why we must include in its description the two muscle planes involved. The pectoralis major muscle belongs on one hand to a fan-shaped muscle group and on the other hand a spiral muscle fiber group.
Its proximal insertion divides the muscle into four portions:
- Clavicular fibers: insert into the inner two thirds of the anterior border of the clavicle;
- Sternal fibers: insert in the outer third of the anterior surface of the sternum. Some fibers cross to the opposite side;
- Chondrocostal fibers: insert in the forelimb and the costal cartilage of the first five or seven ribs;
- Abdominal fibers: correspond to muscle bundles that insert into the sixth and seventh ribs.
The pectoralis major muscle distally ends in the intertubercular groove of the humerus bone, where it shares insertion with latissimus dorsi and teres major muscles. This bony canal, which gives way to the long tendon of the biceps muscle, has two edges and a floor. The teres major inserts on its inner edge and the latissimus dorsi along the floor, leaving the most superficial muscle in the breast region inserted on the outer lip.
At their distal insertion, fibers undergo a helical rotation. For this reason, Gray’s Anatomy includes them in this group, connecting their upper proximal fibers to the lower third of the outer lip of the groove, and the lower proximal fibers to the upper third. By analyzing the connections of the pectoralis major muscle, it can be categorized as:
- Anterior: with the mammary gland and its fascias and supra aponeurotic elements;
- Posterior: the pectoralis minor muscle and rib cage;
- Inferior: two muscles of the anterolateral wall of the abdomen, the oblique on the outside and rectus abdominis on the inside. Some texts on plastic surgery and surgical practice discuss a “step”, an anteroposterior slope between the lower bundles of the pectoralis major and the muscles in question. In cadaveric anatomy, this subtle slope can only be achieved by disinserting the lower bundles of the pectoralis major;
- Exterior: downward, the muscles of the bicipital groove. Outward and upward refer to the deltoid muscle through the deltopectoral groove where the cephalic vein travels upward before draining into the axillary vein.
If the clavicular pectoralis major fibers are disinserted, the cephalic vein can be seen in greater detail piercing Richet’s clavipectoral aponeurosis together with the acromiothoracic artery, one of the arteries involved in the irrigation of this muscle, and the nerve of the pectoralis major. The pectoralis major is mainly irrigated by branches of the acromiothoracic artery and perforating branches from the external mammary artery vessels that are described in the vasculature of the region. Innervation comes from the brachial plexus nerves through the pectoralis major (8,9).
Pectoralis minor and subclavius
These two muscles represent the muscular plane and the deepest constitutive element in the breast region. The subclavius muscle extends from the inferior surface of the clavicle, where it finds a channel with the same name, to the first rib. It is innervated by the subclavian nerve, a branch of the brachial plexus, and its action is to intervene in the descent of the shoulder or to complement the respiratory muscles, depending on whether there is a fixed traction point in the first rib or clavicle, respectively.
The pectoralis minor proximally inserts into the coracoid process of the scapula and from there descends in a fanned fashion, divided into three semitendinosus portions that insert in the third, fourth, and fifth ribs. It is innervated by the pectoralis minor nerve, also a branch of the brachial plexus, and has a complementary action with the subclavian muscle.
The aponeurosis of the subclavian muscle expands to cover the pectoralis minor muscle and forms what is called Richet’s clavipectoral aponeurosis, which is perforated by the aforementioned elements.
Irrigation of the mammary region
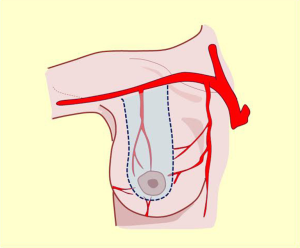
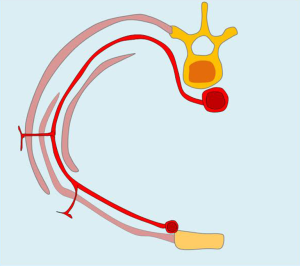
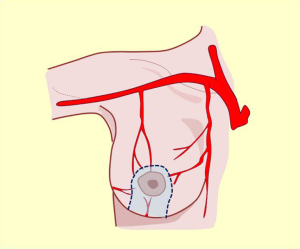
Irrigation of the mammary region was first described in the late nineteenth century by Priet. The literature identifies three distinct arterial territories of irrigation: the axillary artery, internal mammary artery, and intercostal artery (Figure 4A).
Axillary artery
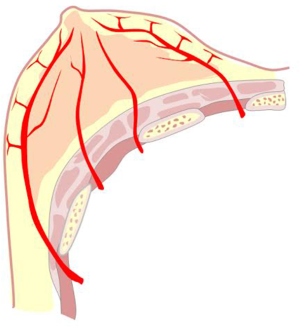
This artery provides significant and defined collateral, used for irrigation in the region. The acromiothoracic artery, the upper thoracic and lower thoracic or external mammary are distributed over the lateral part of the region and anastomose with the branches of the two other pedicle vessels (Figures 2,3,4B,5).
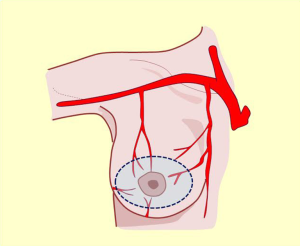
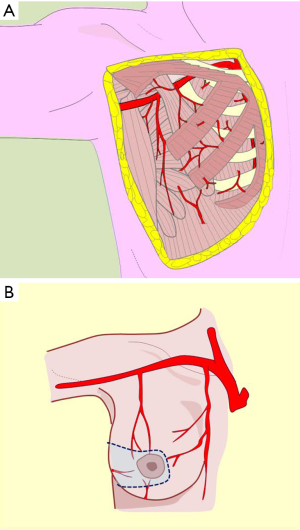
The vascular distribution of the region in a scheme where the pectoralis major muscle was partially resected. We can see the axillary artery continuing to the subclavian artery after passing below the clavicle and subclavian muscle, and three of its collateral branches: the acromiothoracic artery at the level of the pectoralis minor muscle; the lower thoracic or exterior mammary artery, which descends to the serratus anterior muscle and a branch after making a vascular contribution to the latissimus dorsi, goes back to the scapular region; and the thoracodorsal artery. These are tributaries to consider for the selection of pure superior or superior-external pedicles for irrigation and venous drainage of the nipple-areola complex, in reductions of the lower pole (Figures 5,6,7).
Internal mammary artery
The internal mammary arteries, along with the vertebral artery, one of the first collateral branches of the subclavian artery. This artery runs intrathoracically on either side of the breastbone to the diaphragm, where it’s two terminal branches start: one internal or musculophrenic and one external, which pierces the Larrey diaphragmatic hiatus and reaches the abdominal wall as a superior epigastric artery.
Among its many collateral branches, it provides vascularization to the entire internal or medial half of the breast region. These branches run through the intercostal spaces, especially the second, third and fourth, from which they emerge and, after piercing the pectoralis major, are distributed along the medial border of the gland.
These are tributaries relevant to supero-internal pedicle selection for irrigation and venous drainage of the nipple-areola complex, in lower pole reductions or axillary extension (Figures 7A,8) (7,9,10).
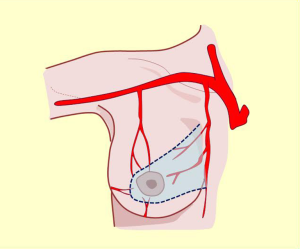
Intercostal arteries
Fairly consistently, the first three intercostal arteries are collateral branches of the cervico-intercostal trunk, a collateral branch of the subclavian artery (7-9). The nine remaining intercostal arteries are collateral branches of the thoracic aorta. The first five or six are responsible for the irrigation of the mammary gland, but secondarily to the internal mammary artery and axillary artery pedicles.
Intercostal perforating branches are distributed on the lateral border of the gland and mammary region (10-16).
These tributaries are relevant to lower posterolateral pedicle or lower nipple-areola complex selection for irrigation in upper pole reductions and super-external (axillary extension) or internal modeling. Non-detachment of the posterior fascia allows the innervation to be preserved (Figures 2,9,10).
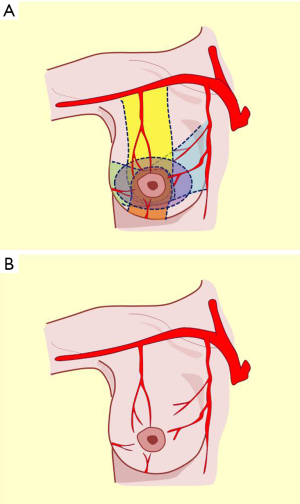
These three vascular pedicles anastomose amongst each other behind the pectoralis major muscle and send perforating branches to the muscle that surrounds the gland from the side to form a supramammary network from which two kinds of branches, originate: glandular and cutaneous. Glandular branches penetrate the breast parenchyma and run along interlobar and interlobular conjunctive septa, later creating a periacinar network. Cutaneous branches become superficial and produce a subdermal network that nourishes all integuments including the nipple areola complex (10,15,17).
This description allows the incorporation of the fifth quadrant concept, proposing that the pure posterior irrigation maintains circulation in the nipple-areola complex (Figure 8, black pedicle). The dermal-subdermal network that nourishes the nipple-areola complex connects peripherally with the entire supramammary network and centrally through perforating vessels called anteromedial costal perforations that traverse the gland from back to front (17). This marked vascular anastomotic arterial and venous disposition is the anatomical basis of flaps election in reduction techniques or modeling the entire breast coverage (Figure 7).
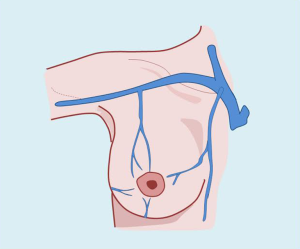
Venous drainage of the breast region
The veins that collect blood from the breast region are run parallel to the arteries the intercostal veins drain into the azygos vein on the right, and into the hemiazygos vein on the left. In some circumstances, the upper intercostal veins may drain directly to the homolateral brachiocephalic trunk (Figure 6).
The subdermal veins form a strong network, intertwined with the blood network, visible in periods of lactation. Areola veins form peripherally, which Testut defines as Haller’s circle. This subcutaneous mammary network communicates upward with the superficial venous network in the neck and down the abdominal wall. The superior veins outline the outer edge of the pectoralis major and the axillary vein drain.
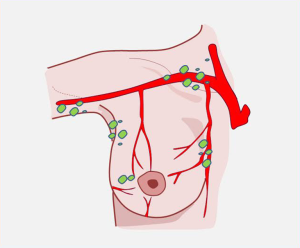
Lymphatic drainage in the breast region
The lymph nodes in the breast region have three distinct pedicles: cutaneous nodes, glandular nodes, and posterior nodes (Figure 11).
Cutaneous nodes
Cutaneous nodes arise from the nipple and areola skin, where they form an extensive lymphatic network called the dermal network in the deep surface of the dermis that contacts another, deeper lymphatic vascular network running through the subcutaneous tissue called the subareolar network (10).
Glandular nodes
Glandular nodes originate from the extralobular (Langhans-Regaud), periacinar space because these nodes dilate widely, they are also called lymph sacs. They are pressed against the lobules and surround the gland, following a centripetal path, and are concentrated at the areola level.
Milk duct nodes
The milk duct nodes run parallel to the milk ducts and flow into the subareolar collection network. Efferent vessels are distinguished into three groups:
The internal group drains the internal mammary nodes and connects with the contralateral group. This connection of the medial mammary groups of both regions is responsible for intermammary metastasis.
Axillary lymph nodes, responsible for receiving lymph from the breast region, correspond to the group of the external mammary artery and the inferior scapular artery. These drain the central lymph group (11).
The central group also receives interpectoral Rother nodes directly. From the central group, lymph runs to the subclavicular nodal group, from which a trunk splits and drains to the right to the thoracic duct and to the left joins the jugular trunk and bronchomediastinal trunk to form the right lymphatic duct that pours into the right brachiocephalic confluence.
The internal mammary chain nodes carry their lymph to the anterior mediastinal nodes and from there to the bronchomediastinal collector, which on the right side drains the thoracic duct and on the left contributes to the formation of the right lymphatic duct.
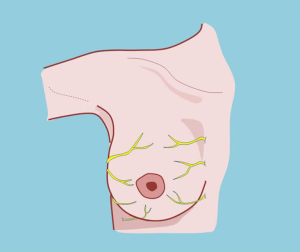
Innervation of the breast region
The sensory innervation of the mammary region originates in the superficial cervical plexus and intercostal nerves (10,11) (Figure 12).
The superficial cervical plexus is represented by the great auricular, occipital, transverse cervical, and supraclavicular nerves. These nerves become superficial at the posterior border of the sternocleidomastoid muscle at the junction of the middle third with the lower third.
The supraclavicular branch is divided into three branches: an external branch called the supraacromial, which is distributed to the superior part of the deltoid region; a medial or supraclavicular branch, which is distributed to the supraclavicular fossa; and an internal or middle branch, called supra-sternal, which is responsible for the innervation of the suprasternal notch.
The second terminal branch of the supraclavicular nerve, which exceeds the limits of the clavicle, is responsible for sensitively innervating the upper quarter of the mammary region.
These four terminal branches of the superficial cervical plexus run supraaponeurotically through the subcutaneous tissue and below the platysma muscle, which sheathes the superficial fascia of the neck (11-13).
The second pedicle intervening in the sensory innervation of the mammary region is represented by the second to the sixth intercostal nerves, which approach the region medially and laterally (10,11).
The intercostal nerves originate from the thoracic spinal nerves. Once this nerve leaves the spinal canal through the foramen, it divides into two branches: one posterior, which is intended for sensory and motor innervation of the dorsal spinal region, and one anterior, which becomes the intercostal nerve.
The intercostal nerves are part of the intercostal neurovascular bundle that runs along the inferior border of the overlying rib in a cephalocaudal fashion: vein, artery, and nerve.
Among the intercostal muscles, the only muscle that accompanies the entire costal extension is the external muscle; the innermost muscle presents an anterior insert, and the internal muscle only occupies the middle part of the extension between the two ribs.
In the posterior arch, the nerve runs together with the bundle into the external intercostal muscle, which then positions itself between the innermost and internal intercostal muscles.
A collateral branch called the parietal lateral perforating branch originates on the lateral side and crosses the innermost and external intercostal muscles, surfacing at the level of the anterior axillary line. In the middle, parasternally, the intercostal nerve ends, perforating the external intercostal muscle, and becomes superficial. These points are located at 1 cm outside the lateral border of the sternum and are called Vailleaux’s points
Preserving the aforementioned branches is of importance when modeling and selecting internal, posterior, and inferior vascular pedicles to preserve innervation of the nipple-areola complex.
Nerve intercostal path
- Intercostal nerve accompanied by the homonymous artery;
- External intercostal muscle;
- Innermost intercostal muscle;
- Internal intercostal muscle;
- Internal mammary artery anastomoses with the intercostal artery (Figure 9).
The innervation of the nipple areola complex derives directly from the anterolateral and anteromedial fourth intercostal nerve (10,11) although the third and fifth intercostal nerves can also contribute to its sensitivity, which explains how innervation can be preserved with the selection of different pedicles.
Notably, these nerves overlap, which could explain why sensitivity remains after some of them have been cut (1,17).
Conclusions
Knowledge of these anatomical considerations will allow us to consider the various techniques of breast reduction with flexibility for selecting different vascular pedicles, depending on the remaining volume, coverage, laxity, and repositioning of the complex. The interpretation of this knowledge is an underlying principle of oncoplastic surgery that aims to model and symmetrize the breast in the pursuit of predictable aesthetic results (Figure 7).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kulkarni D, Beechey-Newman N, Hamed H, et al. Gigantomastia: A problem of local recurrence. Breast 2006;15:100-2. [Crossref] [PubMed]
- Bland KI, Copeland EM. La mama: manejo multidisciplinario de las enfermedades benignas y malignas. Madrid: Ed. Médica Panamericana, 2006.
- Lassus C. A technique for breast reduction. Int Surg 1970;53:69-72. [PubMed]
- Schilder P. Imagen y apariencia del cuerpo humano: estudios sobre las energías constructivas de la psique. México: Paidós, 1989.
- García Porrero J. Anatomía humana. 1st edition. México: Editorial Interamericana, 1995.
- Netter F. Colecciones Netter de ilustraciones médicas’. 1st edition. España: Editorial Masson, 1997.
- Clascá F, Bover R, Burón JA, et al. Anatomía seccional. 1st edition. Madrid: Editorial Elsevier-Masson, 2002.
- Williams PL, Bannister LH. Anatomía de Gray: bases anatómicas de la medicina y la cirugía. 38th edition. Madrid: Harcourt Brace, 1998.
- Bouchet A, Cuilleret J. Anatomía descriptiva, topográfica y fucional. 1st edition. España: Editorial Panamericana, 1997.
- Testut L, Latarjet A. Anatomía humana. 3rd edition. México: Editorial Salvat, 1984.
- Rouviere H, Delmas A. Anatomía humana, topográfica y descriptiva. 9th edition. España: Editorial Masson, 2000.
- Macéa JR, Fregnani JH. Anatomía de la Pared Torácica, Axila y Mama. Int J Morphol 2006;24:691-704.
- Testut L. Jacob O. Tratado de anatomía topográfica con aplicaciones medicoquirúrgicas. 4th edition. México: Editorial Salvat, 1984.
- Gosling J, Harris P, Humpherson J, et al. Atlas de anatomía Humana. 2nd edition. México: Editorial Interamericana – MacGraw-Hill, 1994.
- SkoogT. Atlas de cirugía plástica. 2nd edition. México: Edit Salvat, 1976.
- Rohen JW, YokochiC, Lûtjen-Drecoll E. Atlas de anatomía humana. 6th edition. Madrid: Editorial Elsevier, 2007.
- Bostwick J III. Tratado de mastoplastia. 2nd edition. Madrid: Editorial Amolca, 2001.