BRAF V600E mutation in prognostication of papillary thyroid cancer (PTC) recurrence
Thyroid cancer as a population problem
The increase in the prevalence of thyroid cancer, which is the most common endocrine carcinoma has been observed within the last three decades. It is estimated that by 2019 thyroid cancer will have been the third most prevalent malignant tumor in women in the USA (1). This phenomenon is observed worldwide. For instance, the standardized incidence rate in Poland was 4.9 per 100,000 inhabitants whereas in 1990 it was only 1.0 (2). The fact whether it is a real increase in incidence or the increase in the detection due to the development of more precise diagnostic approaches (sensitive ultrasound, access to fine needle aspiration biopsy) is of lesser significance. As a consequence, the population of newly detected low-advanced thyroid cancers is constantly growing (3). This has one more time resulted in the discussion on optimization of therapeutic strategy. The strategy is related to avoid overtreatment (with unnecessary exposing patients to an increased risk of complications) and undertreatment (i.e., increase in the risk of recurrence and treatment failure) (4-7).
The risk of recurrence in papillary thyroid cancer (PTC)
PTC represents the majority of thyroid carcinomas (~80–90%). It is characterized by excellent prognosis, almost 100% probability of 5-year overall survival (particularly in low-advanced cases). Therefore, in PTC the risk of disease recurrence is most frequently analyzed in relation to the prognosis, i.e., the disease-free survival but not the overall survival unlike in other types of cancers with higher mortality rates (8).
The risk of relapse or persistent disease is related to about 30% of PTC patients and depends, among other things, on the adopted definition of recurrence (8).
The incidence of recurrence depends on whether clinical detection (structural disease) is considered alone or whether it is analyzed together with biochemical recurrence.
From the perspective of a surgeon, structural recurrence seems to be the most significant due to the fact that these patients are most frequently scheduled for surgery. In the context of the therapeutic strategy, the significance of biochemical recurrence cannot be omitted since such patients require a change in routine diagnostic and therapeutic management, e.g., additional treatment with radioactive iodine or increased follow-ups.
Prognostic scales of risk assessment
Currently, much attention is paid to the possibility of patient group selection of different risk of unfavorable outcome in order to match a particular therapeutic approach (8-11).
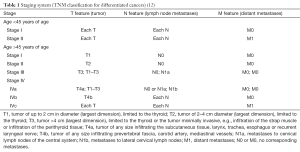
The issue is not new since for many years different prognostic scales have been employed on the basis of which the risk of relapse and the management are prepared. The TNM staging classification, introduced in 1987 by UICC and later accepted by AJCC, is the most common system of cancer classification, including thyroid carcinomas. It allowed to select four stages of clinical advancement, depending on the prognosis. In the case of differentiated thyroid carcinomas, the above classification additionally considers the age factor (12) (Table 1).
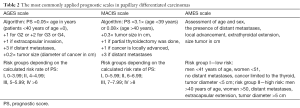
Additionally, some centers individualized their approach to the assessment of prognosis for differentiated thyroid carcinoma by establishing their own prognostic scales, the aim of which is to group patients into adequate risk groups, which allows optimization of treatment modality. The following are the most commonly employed scales: AGES, AMES and MACIS (13-15). All of them consider two major prognostic factors, i.e., age at diagnosis and the presence of distant metastases. Furthermore, the following are analyzed, depending on the scale: tumor size, local advancement, extent of surgery and histological grading (Table 2).
Full table
All of the scales and the assessment of other histological risk factors (e.g., histological subtype, multifocality, angioinvasion, thyroid capsule infiltration) are possible after obtaining the results of postoperative histological examination. Consequently, they do not offer the possibility for planning the extent of surgery and only allow to establish the adjuvant treatment, e.g., 131-iodine therapy.
Therefore, interest in new prognostic and predictive markers known preoperatively has been observed recently. BRAF V600E mutation is an example of such a marker (16).
BRAF mutation—one of the most frequent events in the pathogenesis of PTC
The beginning of 2000 marked the interest in BRAF gene mutation in thyroid cancer. At that time studies on the role of the mutation in other cancers (e.g., melanoma, colon cancer) had been already known (15). BRAF gene is localized on chromosome 7.
It codes cytoplasmic serine/threonine kinase which influences the activation of mitogen-activated pathway kinases (MAPK). BRAF gene mutations activate the MAPK pathway resulting in the intensity of cellular proliferation, inhibition of differentiation and apoptosis. In other words, they lead to a loss of control over the cellular cycle, initiating the development of malignancy as a result. BRAF mutation is one of the most prevalent molecular events in the pathogenesis of PTC in adults (16). Point mutation T1799A is the most common and the most examined mutation of all BRAF gene mutations. It results in the exchanging valine to glutamate at residue 600 near the catalytic center of the protein (BRAF V600E mutation). Finding this mutation is possible not only in postoperative material but also, which is more significant, in fine needle aspiration biopsy material and may be known at diagnosis of PTC (17,18).
The relationship of BRAF mutation with other clinico-pathological risk factors
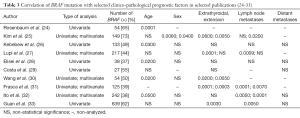
The first reports on the significance of BRAF mutation in the pathogenesis of PTC (19-23) were noted in 2003. Further studies focused on searching the relationship between the mutation and other features of an unfavorable course of the disease (22-31). As early as in 2003 Nikiforova et al. (23) paid attention to the relationship between BRAF mutation and advanced age of patients and also histological subtypes of cancer (classic and tall cell PTC, extracapsular extension and a frequent incidence in patients in III and IV stages. The authors also indicated a more prevalent presence of the mutation in low-differentiated and anaplastic cancers, which suggested the possibility for the mutation to be related to undifferentiation of PTC, which may result in a less favorable prognosis. Other observations were noted in the following years. A number of studies from single centers were reported, which analyzed the prevalence of the mutation and its relationship with other clinico-pathological risk factors (24-33). However, the studies resulted in different, often conflicting results, which were related to differences in methodology, i.e., group selection, group size, time of the follow-up and the type of statistical analyses (uni- and/or multi-variable), the differences in BRAF mutation occurrence in different populations, the methodology of BRAF identification and also lack of the results of validations. Interestingly, initially none of the studies demonstrated the relationship of the mutation with the presence of distant metastases, i.e., the relationship with the strongest factor of an unfavorable prognosis in PTC, most probably due to rare occurrence of metastases (24-33) (Table 3).
Full table
Only in a few studies a relationship between the presence of the mutation and the decrease in disease-free survival was observed (26,28,34). Elisei et al. in 2008 demonstrated the relationship between the presence of BRAF mutation and a decrease in overall and disease-free survival in a group of 102 patients with the mean follow-up of 10 years or longer (28). That study strongly indicated BRAF mutation as an independent, unfavorable prognostic factor in PTC.
Due to a silent biology of PTC and the generally favorable clinical course of this cancer, it is necessary to analyze groups large in size with a long follow-up to observe any significant differences between them. Consequently, no objective prospective, randomized clinical trials were conducted in the past, which could have been the basis for an evidence-based medicine.
Meta-analyses assessing the prognostic significance of BRAF mutation
Four meta-analyses were conducted due to the lack of possibility to run prospective studies for the objective assessment of prognostic significance of BRAF mutation.
In 2007 Lee et al. (35) analyzed 12 papers confirming the relationship between BRAF mutation and the extent of clinical advancement, extrathyroid extension and a histological sub-type. Five years later Li et al. analyzed 32 studies and additionally demonstrated the coexistence of the mutation with the presence of lymph node (LN) metastases, more advanced age and the male sex (36). However, in these papers the relationship between the mutation and the prognosis was not analyzed. Such a study was done by Kim et al. (37). Based on 27 papers these authors also confirmed the relationship between the mutation and extrathyroid extension, LN metastases and the diagnosis of more advanced cancer (according to the TNM). However, in eight papers the relationship between the mutation and a higher risk of persistent disease or recurrence was also confirmed [patients with the mutation had 2.14-fold increased risk of recurrent and persistent disease (95% CI, 1.67–2.74)].
Another meta-analysis by Tufano et al. assessed the correlation between BRAF mutation and recurrence or persistent disease (38). It analyzed 14 papers (the number of patients assessed from 9 countries was 2,470 in total). The authors considered only the reports which discussed the relationship between the recurrence rate and BRAF mutation and other classic clinico-pathological factors (e.g., LN metastases, extrathyroid extension, and more advanced disease; III and IV stages; AJCC). That study confirmed a significantly higher risk of relapse in a group of patients with confirmed BRAF mutation [BRAF (+)] as compared to a group of patients with BRAF wild type [BRAF (−)] (24.9% vs. 12.6%; P<0.00001). However, in the papers included in this analysis, the risk of relapse ranged significantly, i.e., for BRAF (+) patients from 11–40% (mean 26.5%) and for BRAF (−) patients from 2–36% (mean 9.5%).
The results of a multicenter retrospective study of the relationship of BRAF mutation with the prognosis
Xing designed and coordinated a large retrospective international multicentre study to eliminate limitations of meta-analyses for the assessment of the relationship of BRAF mutations with the prognosis in PTC.
The analysis of the relationship of the mutation and the risk of death
The first stage included the assessment of the relationship of BRAF mutation with the risk of death in PTC patients. Xing et al. analyzed 1,849 patients treated in 13 centers in seven countries with the mean follow-up of 33 months (39).
The mortality in these patients was 3% (56/1,849). The mortality rate was significantly lower in patients with BRAF mutation (P<0.001), 45 deaths (5.3%) were reported in a group of BRAF (+) patients and only 11 (1.1%) in a group of BRAF (−) patients. The overall survival was lower in patients in whom the mutation was present. The additional analysis of the overall survival depending on the presence of the mutation and LN metastases demonstrated that BRAF (+) patients with LN metastases constituted the group with the most unfavorable prognosis. The assessment of interactions between BRAF mutation and other risk factors such as LN metastases, distant metastases, age of patients, extrathyroid extension and IV stage of clinical involvement demonstrated a strong relationship between the mutation and each of the above factors except for extrathyroid extension. However, in the multivariate analysis after adding the features of unfavorable disease course such as age, sex, extrathyroid extension, LN metastases or distant metastases, the relationship between BRAF and mortality lost a statistical significance, probably due to a low incidence of deaths.
The analysis of the relationship of the mutation with the risk of recurrence
The next stage was based on the assessment of the relationship between BRAF mutation and the risk of recurrence. Two thousand ninety nine patients from 16 centers and eight countries with the mean follow-up of 36 months were enrolled in the analysis (40). Recurrence was defined altogether as local recurrence, distance recurrence and persistent disease. BRAF mutation was done on postoperative material therefore its status did not have an influence on the treatment applied. Disease recurrence was noted in 16.1% of patients. Recurrence was more frequent in patients with the mutation (20.9%) as compared to BRAF (−) patients (11.6%) (P=0.000). A higher risk of relapse was typical of BRAF (+) patients. The relationship was observed also in the multivariable analysis which considered age, sex, the center from which patients came from, tumor size, extrathyroid extension, LN metastases, multifocality and a histological subtype of the cancer. Disease-free survival was significantly lower in patients with PTC in whom the mutation was diagnosed (log-rank, 28.1; P<0.01). This relationship was also observed in the classic subtype and in follicular subtype of PTC. Additionally, the worst disease-free survival was noted in BRAF (+) patients in whom LN metastases were diagnosed (log-rank, 143.6; P<0.001). A similar observation was related to the coexistence of the mutation with extracapsular infiltration and with the age of patients (>60 years of age) (log-rank, 68.9; P<0.001).
The relationship of BRAF mutation with a higher risk of relapse was also confirmed in low-risk patients (I and II AJCC stages and in microcarcinoma). The study demonstrated that BRAF mutation was an independent unfavorable prognostic factor related to a higher risk of recurrence.
Critical remarks concerning a multicentre retrospective analysis of the relationship between the mutation and disease recurrence
These observations, however, did not dispel the controversy concerning prognostic and predictive significance of BRAF mutation in PTC. After publishing the results of the analysis, the discussion in Journal of Clinical Oncology on the real relationship between the mutation and the cancer relapse was initiated. Bal et al. (41) argued that inaccurate definition of recurrence was used and the definition was applied also for persistent disease. The accusations were also related to a short median follow-up (36 months) and a relatively high incidence of recurrence. These accusations are not fully legitimate (42). In PTC persistent disease is commonly linked with the real recurrence due to the fact that routine follow-ups occur every 6 or 12 months and it is not possible to fully differentiate recurrence from persistent disease as defined in the classic oncological definition. Recurrence is the symptom occurrence after complete remission lasting up to 6 months after the end of treatment.
The majority of relapses occur in the first years of the follow-up, therefore a 3-year follow-up may be considered sufficient, and the reported percentage of recurrence (16%) is within the range given by other authors (9,42).
Furthermore, Yarchoan et al. appreciated the value of the studies of Xing et al. as the largest multicenter retrospective study on prognostic significance of BRAF mutation (43).
These authors argue that current knowledge does not offer enough evidence on the application of BRAF status to the clinical practice. The authors stressed that it may unnecessarily result in anxiety among patients since the significance of BRAF for an unfavorable prognosis is still not strong enough. However, Yarchoan et al. argues that patients from low-risk groups may gain some benefit due to the knowledge on BRAF status (43). It is probable that patients classified into a low-risk group based on the classic prognostic factors and BRAF (−) could be treated conservatively, which is consistent with a current international trend.
Disease recurrence and LN metastases
LN metastases are the most common cause of disease recurrence in PTC. The possibility of a preoperative assessment of the risk of LN metastases would be significant for a surgeon. As a result, patients might be properly qualified for central lymphadenectomy to avoid this prophylactic procedure and an unnecessary increase in incidence risk of postoperative complications (44,45).
The attempt was made to use BRAF mutation in predicting the risk of metastases to cervical LNs (46). Based on uni- and multivariable analyses Joo et al. confirmed the relationship between the mutation and a higher risk of metastases to the central LNs, suggesting that prophylactic central neck dissection should be based on the assessment of BRAF mutation diagnosed in the fine needle aspiration (5). Howell et al. presented similar conclusions and their study confirmed the relationship between BRAF mutation and a risk of metastases to the central LNs (47). These authors expressed the opinion according to which preoperative assessment of BRAF mutation may be used to plan the extent of surgical treatment.
Xing et al. in the multicenter study also confirmed a correlation between the mutation and the risk of LN metastases and a higher risk of recurrence (40). However, some studies did not confirm the presence of such a relationship (7,48,49).
The observed divergence is obviously related to a retrospective nature of the analyses, different group selection (different group size) and different time of follow-up.
It is obvious that well designed prospective study could solve the existing controversies.
Sadly, performing such a study does not seem to be real. Carling et al. assessed that it would be necessary to enroll almost 6,000 patients in the prospective analysis in order to demonstrate the benefit of prophylactic central lymphadenectomy (50).
A critical approach to the practical application of the analysis of BRAF mutation as an independent molecular prognostic and predictive factor
The strongest argument against using BRAF mutation analysis as an independent prognostic and predictive risk factor in patients with PTC is its high prevalence (30–80%) whereas the risk of persistent disease or recurrence is related to about 30% of population (depending on the adopted definition of recurrence).
At present, it seems that BRAF mutation is one of the factors influencing the prognosis and it should be analyzed in correlation with other prognostic factors (51).
The most recent revision of ATA recommendations [2015] is also related to it.
Despite the fact that previously Prescott et al. demonstrated that adding BRAF status to different commonly applied risk scales (AGES, AMES, MACIS, TNM and 2009 ATA initial risk stratification system) improves the possibility of the proper patient classification (52). It allows for a better prediction related to the risk of relapse. The 5-year cumulative recurrence incidence observed by these authors was 20% in BRAF (+) vs. 8% in BRAF (−) patients. BRAF was associated with the time to recurrence when it was added to the following risk-scales: AMES—HR 2.43, MACIS—HR 2.46, TNM—HR 2.51, ATA recurrence risk category—HR 2.44.
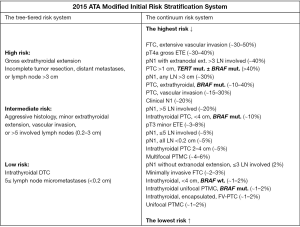
Due to a lack of evident confirmation of a direct influence of mutation on the increase in relapse risk, ATA most recent recommendations do not indicate a routine application of BRAF status for initial risk stratification in differentiated thyroid cancer. However, due to the fact that a clinician may possess such knowledge, ATA demonstrates the continuous risk scale for the assessment of the risk of relapse, considering BRAF and/or TERT status (Figure 1) (53).
Future perspectives and research directions
Currently, researchers are working on determining the role of BRAF mutation in the relapse risk assessment in patients from a low-risk group as indicated by the ATA scale.
Elisei et al. (54) initiated such studies and observed only 3% of structural relapse in patients with thyroid limited carcinoma (T1–T2N0M0). However, in the case of BRAF (+) carcinoma, the recurrence rate was 8% as compared to the population of BRAF (−) 1% (P=0.0003). In the multivariable analysis only BRAF mutation was a predictive factor of persistent disease in a 5-year follow-up. If these observations were confirmed by other studies, the analysis of BRAF mutation for patients with carcinoma limited to the thyroid could result in better selection of low and intermediate risk groups, as suggested by Yarchoan et al. (43).
Recent reports suggest that tumors in which BRAF mutation coexists with other mutations such as TERT, PIK3CA and/or TP53 may be characterized by more aggressive clinical course. It is believed that more accurate tumor prognostication is possible due to a genetic analysis (55-58).
Studies on the formation of sensitive and specific molecular classifiers are being conducted. Such classifiers might help in better assessment of the risk of relapse. Additionally, they might be valuable in the assessment of an unfavorable course of the disease and might be useful in personalized therapeutic strategy. Currently, it seems that the analysis of molecular factors will be one of the major research trends in the following years (59-62).
Summary
The opinion of Puxeddu according to which we cannot use the analysis of BRAF mutation as a single, independent predictive factor in clinical practice seems to be the correct approach (63). However, its usefulness in the context of other molecular and clinico-pathological risk factors cannot be excluded. In the future they may be used to make modern prognostic scales of relapse risk and, as a result, may be applied to plan individual optimal diagnostic and therapeutic strategy for patients with PTC, which is consistent with the most recent ATA recommendations.
Acknowledgements
The authors wish to thank Prof. Barbara Jarząb for invaluable support and general supervision of the manuscript. Additionally, we wish to express our gratitude to Prof. Adam Maciejewski for the improvement of the manuscript. We wish to thank Arkadiusz Badziński MA for the assistance in the translation of the manuscript.
Funding: This work was partially supported by the Polish National Center of Research and Development MILESTONE project—molecular diagnostics and imaging in individualized therapy for breast, thyroid and prostate cancer (grant No. STRATEGMED2/267398/4/NCBR/2015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [Crossref] [PubMed]
- Wojciechowska U, Didkowska J, Zatoński W. Cancer in Poland in 2012. Available online: http://onkologia.org.pl/wp-content/uploads/Rok2012.pdf
- Aschebrook-Kilfoy B, Schechter RB, Shih YC, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev 2013;22:1252-9. [Crossref] [PubMed]
- Pelizzo MR, Dobrinja C, Casal Ide E, et al. The role of BRAF(V600E) mutation as poor prognostic factor for the outcome of patients with intrathyroid papillary thyroid carcinoma. Biomed Pharmacother 2014;68:413-7. [Crossref] [PubMed]
- Joo JY, Park JY, Yoon YH, et al. Prediction of occult central lymph node metastasis in papillary thyroid carcinoma by preoperative BRAF analysis using fine-needle aspiration biopsy: a prospective study. J Clin Endocrinol Metab 2012;97:3996-4003. [Crossref] [PubMed]
- Xing M. BRAF mutation in papillary thyroid microcarcinoma: the promise of better risk management. Ann Surg Oncol 2009;16:801-3. [Crossref] [PubMed]
- Danilovic DL, Lima EU, Domingues RB, et al. Pre-operative role of BRAF in the guidance of the surgical approach and prognosis of differentiated thyroid carcinoma. Eur J Endocrinol 2014;170:619-25. [Crossref] [PubMed]
- Omry-Orbach G. Risk Stratification in Differentiated Thyroid Cancer: An Ongoing Process. Rambam Maimonides Med J 2016.7. [PubMed]
- Tuttle RM, Tala H, Shah J, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010;20:1341-9. [Crossref] [PubMed]
- Malandrino P, Latina A, Marescalco S, et al. Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J Clin Endocrinol Metab 2011;96:1703-9. [Crossref] [PubMed]
- Krajewska J, Jarząb M, Czarniecka A, et al. Ongoing risk stratification for differentiated thyroid cancer (DTC) - stimulated serum thyroglobulin (Tg) before radioiodine (RAI) ablation, the most potent risk factor of cancer recurrence in M0 patients. Endokrynol Pol 2016;67:2-11. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. editors. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- Cady B. Hayes Martin Lecture. Our AMES is true: how an old concept still hits the mark: or, risk group assignment points the arrow to rational therapy selection in differentiated thyroid cancer. Am J Surg 1997;174:462-8. [Crossref] [PubMed]
- Fraker DL, Skarulis MC, LiVolsi VA. Cancer of endocrine system. Thyroid tumors. In: DeVita VT Jr, Hellman S, Rosenberg SA. Editors. Cancer. Principles and practice of oncology. Philadelphia: J.B. Lippincott, 2001.
- Handkiewicz-Junak D, Czarniecka A, Jarzab B. Molecular prognostic markers in papillary and follicular thyroid cancer: Current status and future directions. Mol Cell Endocrinol 2010;322:8-28. [Crossref] [PubMed]
- Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007;28:742-62. [Crossref] [PubMed]
- Nam JK, Jung CK, Song BJ, et al. Is the BRAF(V600E) mutation useful as a predictor of preoperative risk in papillary thyroid cancer? Am J Surg 2012;203:436-41. [Crossref] [PubMed]
- Xing M, Clark D, Guan H, et al. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol 2009;27:2977-82. [Crossref] [PubMed]
- Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003;95:625-7. [Crossref] [PubMed]
- Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 2003;22:4578-80. [Crossref] [PubMed]
- Xu X, Quiros RM, Gattuso P, et al. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res 2003;63:4561-7. [PubMed]
- Fukushima T, Suzuki S, Mashiko M, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene 2003;22:6455-7. [Crossref] [PubMed]
- Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 2003;88:5399-404. [Crossref] [PubMed]
- Rosenbaum E, Hosler G, Zahurak M, et al. Mutational activation of BRAF is not a major event in sporadic childhood papillary thyroid carcinoma. Mod Pathol 2005;18:898-902. [Crossref] [PubMed]
- Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364-8. [Crossref] [PubMed]
- Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 2007;246:466-70; discussion 470-1. [Crossref] [PubMed]
- Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab 2007;92:4085-90. [Crossref] [PubMed]
- Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943-9. [Crossref] [PubMed]
- Costa AM, Herrero A, Fresno MF, et al. BRAF mutation associated with other genetic events identifies a subset of aggressive papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2008;68:618-34. [Crossref] [PubMed]
- Wang Y, Ji M, Wang W, et al. Association of the T1799A BRAF mutation with tumor extrathyroidal invasion, higher peripheral platelet counts, and over-expression of platelet-derived growth factor-B in papillary thyroid cancer. Endocr Relat Cancer 2008;15:183-90. [Crossref] [PubMed]
- Frasca F, Nucera C, Pellegriti G, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer 2008;15:191-205. [Crossref] [PubMed]
- Ito Y, Yoshida H, Maruo R, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J 2009;56:89-97. [Crossref] [PubMed]
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab 2009;94:1612-7. [Crossref] [PubMed]
- Fugazzola L, Mannavola D, Cirello V, et al. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf) 2004;61:239-43. [Crossref] [PubMed]
- Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 2007;110:38-46. [Crossref] [PubMed]
- Li C, Lee KC, Schneider EB, et al. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab 2012;97:4559-70. [Crossref] [PubMed]
- Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 2012;118:1764-73. [Crossref] [PubMed]
- Tufano RP, Teixeira GV, Bishop J, et al. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine (Baltimore) 2012;91:274-86. [Crossref] [PubMed]
- Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013;309:1493-501. [Crossref] [PubMed]
- Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 2015;33:42-50. [Crossref] [PubMed]
- Bal C, Ballal S. Is There Any True Association Between BRAF V600E Mutation and Recurrence, Particularly in Low-Risk, Papillary Thyroid Cancer? J Clin Oncol 2015;33:2481. [Crossref] [PubMed]
- Xing M. Reply to C. Bal et al. J Clin Oncol 2015;33:2483-4. [Crossref] [PubMed]
- Yarchoan M. LiVolsi VA, Brose MS. BRAF mutation and thyroid cancer recurrence. J Clin Oncol 2015;33:7-8. [Crossref] [PubMed]
- Czarniecka A, Jarzab M, Krajewska J, et al. Prognostic value of lymph node metastases of differentiated thyroid cancer (DTC) according to the local advancement and range of surgical excision. Thyroid Res 2010;3:8. [Crossref] [PubMed]
- Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [Crossref] [PubMed]
- Alzahrani AS, Xing M. Impact of lymph node metastases identified on central neck dissection (CND) on the recurrence of papillary thyroid cancer: potential role of BRAFV600E mutation in defining CND. Endocr Relat Cancer 2013;20:13-22. [Crossref] [PubMed]
- Howell GM, Nikiforova MN, Carty SE, et al. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol 2013;20:47-52. [Crossref] [PubMed]
- Niederer-Wüst SM, Jochum W, Förbs D, et al. Impact of clinical risk scores and BRAF V600E mutation status on outcome in papillary thyroid cancer. Surgery 2015;157:119-25. [Crossref] [PubMed]
- Chakraborty A, Narkar A, Mukhopadhyaya R, et al. BRAF V600E mutation in papillary thyroid carcinoma: significant association with node metastases and extra thyroidal invasion. Endocr Pathol 2012;23:83-93. [Crossref] [PubMed]
- Carling T, Carty SE, Ciarleglio MM, et al. American Thyroid Association design and feasibility of a prospective randomized controlled trial of prophylactic central lymph node dissection for papillary thyroid carcinoma. Thyroid 2012;22:237-44. [Crossref] [PubMed]
- Czarniecka A, Kowal M, Rusinek D, et al. The Risk of Relapse in Papillary Thyroid Cancer (PTC) in the Context of BRAFV600E Mutation Status and Other Prognostic Factors. PLoS One 2015;10:e0132821. [Crossref] [PubMed]
- Prescott JD, Sadow PM, Hodin RA, et al. BRAF V600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery 2012;152:984-90. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Elisei R, Viola D, Torregrossa L, et al. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab 2012;97:4390-8. [Crossref] [PubMed]
- Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 2014;32:2718-26. [Crossref] [PubMed]
- Huang Y, Liao D, Pan L, et al. Expressions of miRNAs in papillary thyroid carcinoma and their associations with the BRAFV600E mutation. Eur J Endocrinol 2013;168:675-81. [Crossref] [PubMed]
- Song YS, Lim JA, Choi H, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 2016;122:1370-9. [Crossref] [PubMed]
- Lee SE, Hwang TS, Choi YL, et al. Prognostic Significance of TERT Promoter Mutations in Papillary Thyroid Carcinomas in a BRAF(V600E) Mutation-Prevalent Population. Thyroid 2016;26:901-10. [Crossref] [PubMed]
- Wu JX, Young S, Hung ML, et al. Clinical Factors Influencing the Performance of Gene Expression Classifier Testing in Indeterminate Thyroid Nodules. Thyroid 2016;26:916-22. [Crossref] [PubMed]
- Kloos RT, Barth NM. Gene Expression Classifier Testing and the Surgical Decision-Making Process for Patients With Thyroid Nodules. JAMA Otolaryngol Head Neck Surg 2016;142:806-7. [Crossref] [PubMed]
- Scott E, Learoyd D, Clifton-Bligh RJ. Therapeutic options in papillary thyroid carcinoma: current guidelines and future perspectives. Future Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Wylie D, Beaudenon-Huibregtse S, Haynes BC, et al. Molecular classification of thyroid lesions by combined testing for miRNA gene expression and somatic gene alterations. J Pathol Clin Res 2016;2:93-103. [Crossref] [PubMed]
- Puxeddu E, Filetti S. BRAF mutation assessment in papillary thyroid cancer: are we ready to use it in clinical practice? Endocrine 2014;45:341-3. [Crossref] [PubMed]