Chylous ascites after resection of giant adrenocortical carcinoma
Introduction
Chylous ascites is a clinical state characterized by the accumulation of chyle in the peritoneal cavity primarily caused by diseases affecting the abdominal or retroperitoneal lymphatic glands. Postoperative chylous ascites (PCA) is a very rare complication caused by unobserved interruption of the major retroperitoneal lymphatic channels and by formation of lymphoperitoneal fistula following retroperitoneal and mediastinal surgery (1). Although PCA is rarely observed, the need to diagnose and treat the condition as a clinical state has been increasing in importance with the increased number of wide resections and lymph node dissections being performed during abdominal surgery (2). Increased attention to PCA is warranted to due to its consequences, namely prolonged duration of hospitalization and increased medical costs arising from the need to treat the malnutrition, dehydration, immunosuppression, and/or septic complications associated with the condition (2). Despite the importance of increasing knowledge of PCA, very few case studies have described the nature of PCA complications that develop after adrenalectomy and their successful treatment (3-5). To help fill this research gap, we describe our diagnosis and successful treatment of a case of giant adrenocortical carcinoma and the PCA complications that developed after resection.
Case presentation
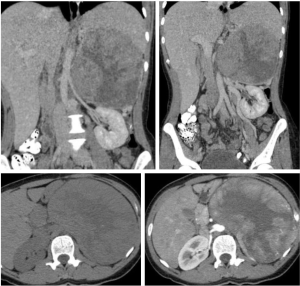
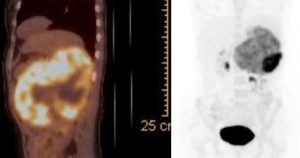
A 38-year-old female patient was admitted to our emergency clinic complaining of abdominal pain. During physical examination, an immobile, solid mass was palpated in the upper left abdominal region. Computerized tomography (CT) and ultrasonography (USG) revealed a retroperitoneal mass of approximately 20 cm × 18 cm × 15 cm lying inferiorly in the left suprarenal region. The mass showed the marked effects of exertion of pressure on the adjacent vascular structures (the renal, splenic, ovarian, and inferior mesenteric veins), causing widespread development of venous collaterals (Figure 1), as well as on the organs (the pancreas, left kidney, and spleen), but no signs of invasion. Review of the results of blood and urine analyses (1 mg-dexamethasone suppression test, plasma or urine free metanephrines and plasma aldosterone concentration to plasma renin activity ratio) were all normal limits, so then it resulted in a pre-diagnosis of nonfunctional suprarenal mass. Positron emission computerized tomography (PET-CT) revealed a wide mass showing intense activity trapping (SUVmax: 24.4; Figure 2). Based on these findings, malign nonfunctional adrenal mass was diagnosed and left total adrenalectomy was performed (Figures 3).

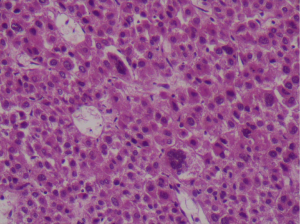
During postoperative follow-up, fever and abdominal signs were not observed, and oral nutrition was begun on day 1. Upon observation, that approximately 500 cc of chylous ascites had drained on day 2, oral nutrition was stopped and total parenteral nutrition (TPN) was started. The amount of fluid drained declined to 300 cc on day 3 and to 0 cc on day 4. After abdominal USG revealed no pathological signs, TPN was stopped and oral nutrition was resumed. When no further drainage was observed following resumption of oral nutrition, the drain tube was removed and the patient was discharged from the hospital. Gross examination of the specimen revealed a 1,550 g, 22 cm × 12 cm × 11 cm, round, partly capsulated, friable mass. The sections showed a variegated appearance with tan-yellowish nodules and necrotic areas. Histologically, the tumor was composed of diffuse and sheetless cells showing marked nuclear pleomorphism with unusual multinucleated giant cells with macronucleoli and a large asidophilic cytoplasm (Figure 4). Extensive necrosis; capsular, perineural, and lymphovascular invasion; and 8 mitotic figures per 10 high-power-field were observed. Immunohistochemical staining with inhibin and pancytokeratin focally revealed the tumor cells to be negative for synaptophysin, chomogranin, vimentin, CEA, EMA, Melan-A, and CD10 and the Ki67 proliferation index to be 15–20%. Based on these findings, the tumor was diagnosed as adrenocortical carcinoma. Adjuvant mitotane treatment for 2 years was planned and 6 months after surgery patient was in a good clinical condition without any identified metastasis.
Discussion
Retroperitoneal and mediastinal surgery may lead to formation of PCA secondary to direct damage to lymphatic vessels. PCA may appear within 1 week following surgery due to disruption of lymphatic vessels or weeks or months after surgery due to development of adhesions or extrinsic compression of lymphatic vessels (6). Factors that indicate the need for more extensive surgery or extended manipulation of the lymphatic vessels are predictive for PCA. Based on a review of the data, Weniger et al. reported that the incidence of PCA increases in extended resections and is correlated with the number of lymph nodes harvested (2). They also identified concomitant vascular resection or manipulation of the paraaortic area, retroperitoneal tumor invasion, and increased blood loss as other risk factors. Regarding treatment, Joanny et al. strongly recommended meticulous dissection and permanent use of hemoclips and/or suture ligatures, irrespective of the type of surgery performed (3).
Lymphatics of the adrenal gland are located in its capsule and drain either into the ductus thoracicus via the regional lymph nodes or directly into the cisterna chyli (5). According to Weniger et al., clinically significant PCA is defined as a daily minimum volume of non-purulent fluid in the abdominal cavity or drained fluid of a milky appearance with a triglyceride level of >110 mg/dL (2). Determining the white blood cell count and performing a microbiological culture are essential to exclude infection, and detailed analysis of drained fluid may be helpful in suspected cases. In their microscopic investigation of fluid rich in triglycerides and protein using Sudan III staining, Joanny et al. observed lipid globules and leukocytes, most of which were lymphocytes (3).
Although initial management of PCA is conservative, its success rate is considerably high, especially in cases treated by TPN (2). In cases in which TPN is contraindicated or when more than <200 mL is drained, nutrition with medium-chain triglycerides (MCTs) is recommended (2). Administration of octreotide, an analog of somatostatin, may shorten duration of hospitalization when used in conjunction with TPN or MCT nutrition, and should be considered in cases of severe PCA (7). In cases showing resistance to conservative treatment, interventional or surgical procedures may be necessary. One alternative treatment especially effective with patients with a daily chylous drainage of <500 mL is bipedal lymphangiography with lipiodol, which has been reported to yield a chylous leakage occlusion rate of up to 70% (8). When conservative treatment or treatment with lymphangiography is unsuccessful, direct ligation of disrupted vessels by open surgery or implantation of a peritoneovenous shunt may be necessary (3).
There are limited numbers of cases describing PCA complication after adrenal gland surgery (Table 1). Another paper demonstrated early PCA diagnosed by the appearance and analysis of milky fluid on drain sac (3) as our case while in others it was diagnosed by CT scans in late period (3,9,10) and drained percutaneously (4,5,9,10). One patient is died due to extensive metastatic involvement (5) while in others conservative treatment is successfully applied (3,4,9,10). In our case, PCA was observed on postoperative day 2 and after TPN administration it was discontinued on postoperative day 4. Amongst the reported case reports, our case has the largest mass size and PCA development may be correlated with the size of the mass, with a larger mass exerting greater pressure on vascular structures.

Full table
Conclusions
The increasing incidence of PCA development during abdominal surgery, especially after resection of giant adrenal masses, has highlighted the need to prevent this postoperative condition. Use of suturing during dissection, when possible, appears to decrease the risk of PCA development. In cases in which prevention is not possible, early, reliable diagnosis and prompt treatment is important for successful resolution of both the PCA and the primary condition.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient’s next of kin for publication of this manuscript and any accompanying image.
References
- Leibovitch I, Mor Y, Golomb J, et al. The diagnosis and management of postoperative chylous ascites. J Urol 2002;167:449-57. [Crossref] [PubMed]
- Weniger M, D'Haese JG, Angele MK, et al. Treatment options for chylous ascites after major abdominal surgery: a systematic review. Am J Surg 2016;211:206-13. [Crossref] [PubMed]
- Joanny G, Celia A, Zeccolini G, et al. Chylous ascites following laparoscopic adrenalectomy: case report and literature review. Arch Ital Urol Androl 2010;82:186-8. [PubMed]
- Qi J, Gu ZQ, Chen F, et al. Management of postoperative chyloretroperitoneum in adults. Singapore Med J 2009;50:e338-41. [PubMed]
- Türkbey B, Wahbeh H, Akpinar E. Abdominal chyloma: computed tomography findings. Turk J Gastroenterol 2011;22:641-2. [Crossref] [PubMed]
- Cárdenas A, Chopra S. Chylous ascites. Am J Gastroenterol 2002;97:1896-900. [Crossref] [PubMed]
- Kuboki S, Shimizu H, Yoshidome H, et al. Chylous ascites after hepatopancreatobiliary surgery. Br J Surg 2013;100:522-7. [Crossref] [PubMed]
- Alejandre-Lafont E, Krompiec C, Rau WS, et al. Effectiveness of therapeutic lymphography on lymphatic leakage. Acta Radiol 2011;52:305-11. [Crossref] [PubMed]
- Turkbey B, Ozer C, Akinci D, et al. Abdominal chyloma: CT findings and percutaneous drainage. Cardiovasc Intervent Radiol 2009;32:601-2. [Crossref] [PubMed]
- De Sousa P, Viart L, Petit J, et al. Chylous ascites after trans-peritoneal laparoscopic adrenalectomy: anatomical distribution of lymph nodes and management. Prog Urol 2010;20:385-8. [Crossref] [PubMed]


