
The deep inferior epigastric perforator flap: where we started and where we are now
Introduction
Background
The deep inferior epigastric perforator (DIEP) flap was described in 1989 and is generally considered the preferred flap for autologous breast reconstruction (1). As with all plastic surgery techniques, modifications and innovations will change the nature of a given technique with the goal being to improve outcomes and reduce complications. This has certainly been the case with the DIEP flap that several surgeons began performing in 1990s. Prior to that, the transverse rectus abdominis musculocutaneous (TRAM) flap, either pedicle or free, was the preferred flap for autologous breast reconstruction. Since 1999, the author has performed over 2,500 DIEP flaps and has witnessed numerous modifications and refinements in order to improve outcomes. Many of these refinements were based on personal experience and other modifications were based on the experience of others who had presented or published their work (2-6). All surgeons have their particular nuances regarding the surgical technique for DIEP flaps; however, the common strategy has remained constant and is to harvest the adipocutaneous tissues of the anterior abdominal wall, to retain the continuity, width and innervation of the rectus abdominis muscle, and to achieve excellent breast and abdominal aesthetics.
Rationale and knowledge gap
Since the early 2000s, the number of plastic surgeons performing DIEP flap reconstruction has steadily increased and the number of DIEP flaps performed for autologous breast reconstruction has exponentially increased. During the 1990s, there were a small number of microsurgeons in the USA that performed DIEP flaps on a regular basis. However, as the demand for perforator-based reconstruction increased, DIEP flap education in residency programs worldwide increased to the point today where DIEP flap surgeons are abundant and located in most large metropolitan cities throughout the world. A review of the 2020 American Society of Plastic Surgeons procedural statistics demonstrated that 23,324 DIEP flaps were performed in the USA representing a 125% increase from the 10,338 that were performed in 2019 (7). This increase can be attributed to a variety of factors that include concerns associated with silicone gel breast implants such as lymphoma, breast implant illness, and the fact that they are not lifetime devices. DIEP flaps, on the other hand, constitute a natural reconstruction that lasts forever with minimal morbidity.
Objective
The purpose of this manuscript is to review many of the modifications and to review where we were and where we currently are with the DIEP flap for breast reconstruction. This will be subdivided into preoperative, intraoperative and postoperative considerations.
Preoperative
The preoperative preparation for DIEP flap breast reconstruction has evolved over the past 20 years. My standard algorithm for breast reconstruction is based on body habitus and breast volume requirement such that about 20% of patients are ideally suited for a DIEP flap only, 20% of patients are ideal candidates for prosthetic reconstruction only and 60% of patients can have a DIEP or prosthetic reconstruction (8,9). Thus, based on personal experience, a DIEP flap is possible in about 80% of women who are interested in breast reconstruction following mastectomy.
A common belief amongst many microsurgeons during the early years of performing DIEP flaps is that it was best performed in an institutional setting with ample ancillary staff to ensure that these operations proceed smoothly. From 1997–2017, my practice was based in a university setting where I worked with residents and fellows in training. We had a well-trained staff in the clinic and in the operating room and the ability to care for these patients postoperatively in an intensive care unit (ICU). My personal opinion at that time was that these complex operations were ideally suited for that environment; however, over time we realized that university hospitals and ICU settings were not always the ideal environment. We were able to manage and care for all of our postoperative DIEP flap patients in specialized nursing stations at both university-based hospitals where I worked. Since 2017, I have been in private practice and now believe that the most important factor to improve outcomes is to have experienced and competent well-trained microvascular surgeons working as a team. In contrast to the teams that were used prior to 2017 that included a faculty member and resident or fellow, it is our new strategy that all microvascular breast reconstructions be performed with two skilled and experienced microvascular surgeons. This is based on recent studies demonstrating that operative time is reduced, complications are fewer and that outcomes are improved using a two-team (microsurgeon) approach (10). Traditionally, these operations were performed at plastic surgery teaching hospitals in which there was a noted increase in operative time, ischemia time, and complications.
Historically, many oncologists were of the opinion that delayed autologous reconstruction was preferred to immediate based on fear of recurrence and reducing operative time. However, over time, the benefits of immediate autologous reconstruction have been realized as patients are able to go to sleep with their breasts and wake up from anesthesia with an optimally shaped DIEP flap. In many patients, tertiary reconstruction with a DIEP flap is performed in the event of a failed prosthetic reconstruction or when radiation therapy has damaged the soft tissues of the chest obviating the ability to use prosthetic devices. In all of these situations, the DIEP flap has become the preferred flap for autologous reconstruction and has the ability to create an optimally shaped, contoured and positioned breast.
During the initial period of performing DIEP flaps, operative time was typically 7–10 hours for bilateral reconstruction and 5–6 hours for unilateral. Today, using the two-team approach, operative time is typically 4–6 hours for bilateral reconstructions and 3–4 hours for unilateral reconstruction. This has been corroborated by the experience of others that also utilize the two-team approach (11,12). Haddock has evaluated flap harvest times and demonstrated 54.8 minutes for faculty surgeons operating together, 98.3 minutes for a senior resident or fellow working with a faculty member, and 178.8 minutes for a supervised chief resident (P<0.001) (11). Personal experience assessing flap survival demonstrated a 97% success prior to the two-team approach and 99% success with the two-team approach. It is now well recognized and appreciated that as surgical teams improve in quality, the safety and efficacy of DIEP flap reconstruction is optimized.
Another relatively recent and important aspect in the preoperative planning of DIEP flaps is the ability to assess the vascular architecture of the anterior abdominal wall using computerized tomographic angiography (CTA) or magnetic resonance angiography (13,14). Prior to imaging, most surgeons relied on their intraoperative dissection skills to determine the location and caliber of the perforating vessels. With the advent of perforator imaging, surgeons can now demonstrate the exact location of the perforators as they perforate the anterior rectus sheath and ascend towards the skin. In addition, the patency and location of the deep inferior epigastric vessels can be demonstrated as type 1 (single vessel), type 2 (double branching pattern), and type 3 (triple branching pattern). In a recent randomized controlled trial focused on CTA versus no CTA, it was demonstrated that preoperative CTA analysis of perforators decreases flap harvest and overall operative time with equivalent postoperative outcomes (13).
Intraoperative
There have been many significant technological advancements that have facilitated our ability to perform DIEP flap reconstruction When I and many microvascular surgeons began performing DIEP flaps in the 1990s, a hand-sewn arterial and venous anastomosis was common (15,16) (Figure 1). The venous coupler has had a significant positive impact on improving venous patency and decreasing ischemia times. Fitzgerald O'Connor et al. have demonstrated good success using the coupler for venous anastomosis (17). They were able to demonstrate a take back rate of 12.7% and a flap success rate of 99.3%. The failure of the coupler device was less than 1.4% whereas the failure of a hand-sewn anastomosis was 3.57% (P=0.001). This is in line with my experience.
The choice of recipient vessels for DIEP flap reconstruction includes the thoracodorsal, the internal mammary as well as perforators of the internal mammary artery and vein. Early in our experience, the thoracodorsal vessels were used; however, early in our experience, we transitioned to the internal mammary artery and vein. Reasons for this included a higher flow vascular system, optimal location that allows for improved positioning of the flap on the chest wall and the ease of dissection (18). Another option is to use the perforators off of the internal mammary vessels. In some cases, these may be sufficient and obviate the need to remove the medial cartilaginous segment of the 3rd or 4th rib. Benefits of this include less dissection time and possibly less postoperative pain.
Another advancement related to DIEP flaps as well as flaps in general is the ability to assess perfusion within the flap while in the operating room (14). From a historical perspective, flap perfusion has been assessed clinically by documenting arterial and venous bleeding from the distal edge of the flap, flap color to assess hyperemia or congestion, and flap turgor. Although useful, it is not an accurate predictor of skin or fat necrosis. The use of indocyanine green (ICG) associated with tissue perfusion analysis has been used intraoperatively since 2009. The benefit of ICG angiography is that the surgeon is able to assess the “real-time” perfusion of the DIEP flap and determine the extent of perfusion within the various zones (19). In addition, it can be used to assess patency of the arterial and venous anastomosis by being able to visualize the flow within the artery and vein. Another application is to demonstrate perfusion within the mastectomy skin flap and the nipple areolar complex following nipple or skin sparing mastectomy. Fat necrosis is a potential factor with autologous reconstruction and may be the result of arterial insufficiency resulting in poor distal tissue perfusion and distal tissue necrosis as well as venous insufficiency resulting in tissue congestion and focal tissue necrosis. In a recent randomized clinical trial evaluating the efficacy of ICG angiography, 2 cohorts were compared: Cohort one (n=27) had excision of tissue based on clinical assessment and cohort two (n=24) had excision based on ICG angiography (20). The authors demonstrated a fat necrosis rate of 59.3% in cohort 1 and 8.3% in cohort 2. The reoperation rate was 14.8% in cohort 1 and 0 in cohort 2. The authors concluded that perfusion rates, patient satisfaction and quality of life were enhanced. In another study evaluating ICG perfusion in DIEP flaps, Yoo et al. demonstrated no difference in fat necrosis rates comparing patient who had and did not have ICG perfusion; however, they were able to demonstrate a relationship between the incidence of fat necrosis and body mass index as a continuum (P=0.001) and when categorized as greater than 35 (P=0.038) (21).
Another modification in my personal technique for harvesting DIEP flap relates to the number of perforators included in the flap. Early in my experience, the DIEP flap was typically elevated on a single perforator. At that time, the decisions were made regarding whether to remove or not to remove a small segment of the rectus abdominis muscle (9,22). In a review of my first 163 free TRAM or DIEP flaps performed between 1997–2000, 85% of the DIEP flaps were raised on a single perforator (8). This was based on ensuring that a dominant perforator was present and also because the dissection of a large caliber perforator was safer and easier. Over the years, as microsurgical breast reconstruction has flourished and we have become more comfortable with dissection techniques, the number of perforators included with DIEP flaps has increased. I typically will include 2–3 perforators with the majority of DIEP flaps. The main reasons for this are to include more angiosomes within the flap to enhance perfusion throughout the zones include. The second reason is that inclusion of more perforators will minimize the risk of pedicle twisting. Having two or more perforators will maintain the vascular pedicle in the proper orientation proximal to the point of connection of the two branches (Figure 2). A single pedicle is at increased risk of twisting anywhere along its entire length (Figure 3). The drawback to the inclusion of more than one perforator is increased trauma to the rectus abdominis muscle because of the additional intramuscular dissection; however, it is this authors opinion the benefits of added perfusion to the flap outweigh the minor disadvantage of increased trauma to the rectus abdominis muscle. It is recognized that there is controversy regarding the number of perforators to include and that the decision will ultimately be based on the vascular anatomy and surgeon preference. In a systematic review spanning 30 years and 28 studies, Bhullar et al. demonstrated that inclusion of 2–4 perforators had the lowest rates of fat necrosis (23). They also demonstrated that medial row perforators had a broad zone of perfusion, whereas lateral row perforators had a narrow zone of perfusion.
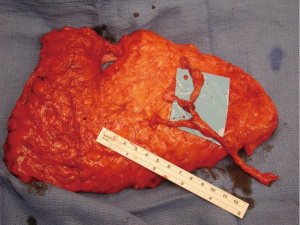
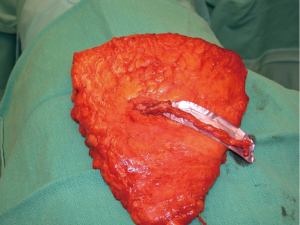
Additional important factors with the DIEP flap harvest include the length of the vascular pedicle and the preservation of the lateral intercostal nerves. With all DIEP flap perforator dissections, it is imperative to preserve the lateral intercostal nerves as they enter the rectus abdominis muscle. Failure to do so will result in loss of muscle function that is far more destructive than any degree of muscle dissection. With regard to pedicle length, there are various schools of thought. Some believe that a short 5–6 cm pedicle is sufficient and will terminate the dissection when this length is achieved. Others believe that the dissection of the inferior epigastric artery and vein should continue to its origin at the iliac vessels because the caliber of the vessels closely correlates with that of the recipient internal mammary vessels. It is the opinion of this author that outcomes are improved when the “micro” is as “macro” as possible. The length of the rectus abdominis myotomy has minimal impact on pain or outcomes.
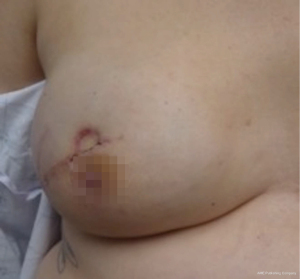
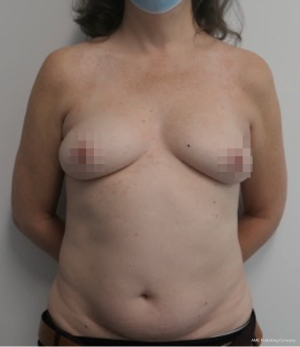
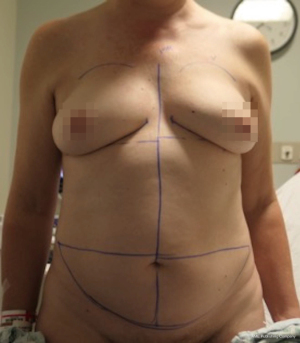
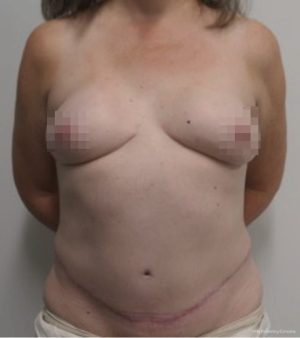
Nipple sparing mastectomy (NSM) has been a significant advancement that had had a tremendous impact on aesthetic outcomes and patient satisfaction (24). In properly selected patients, the entire skin envelope is usually preserved and in the event of a DIEP flap, postoperative monitoring is a consideration. Historically, what was performed was to leave a small cutaneous island of the DIEP flap exteriorized for evaluation based on color and Doppler signal. Before de-epithelizing the flap, the location of the Doppler signal was noted and a small skin island was preserved so that the flap could be properly monitored (Figure 4). Today, however, we rarely exteriorize a skin territory because the Doppler signal from the DIEP flap easily transmits through the intact mastectomy skin flap. The benefit of this is to minimize scars on the breast and to reduce secondary operations. Figures 5-7 illustrate a patient following NSM and DIEP flap reconstruction in which the flap was buried under the mastectomy skin.
Lymphedema has always been a risk following mastectomy with lymphadenectomy. This may be due to disruption of the normal lymphatic pathways due to excision of lymph nodes, disruption in the lymphatic pathways and scar tissue formation in the axilla. From a historic perspective, correction of mastectomy related lymphedema has been with the use of independent vascularized lymph node transfer or lymphovenous bypass. Given the complexity of surgical management of lymphedema, recent work has focused on management in the setting of DIEP flap reconstruction. The superficial inguinal lymph nodes are in close proximity to the inferolateral aspect of the flap. The traditional DIEP flap is harvested without inclusion of any lymph nodes. Thus, in patients with lymphedema, the DIEP flap can be harvested in continuity with the superficial inguinal lymph nodes that can be positioned into the axillary lymph node basin during DIEP flap inset. In a recent study evaluating the quality of life. Winters et al. reported on 64 patients that had DIEP flap reconstruction with lymph node inclusion (25). Of these patients 45 completed a questionnaire reporting on outcome. The authors demonstrated a 69% decrease in physiotherapy requirements, 63% of patients were able to decrease compression garment usage, and 6 of 7 patients with recurrent skin infections had a reduction in the incidence. As with other studies, these authors were not able to demonstrate a significant reduction in volume between the affected and non-affected arms (407 vs. 406 mL, P=0.988).
Postoperative
During the early days of DIEP flap reconstruction, hospitalization typically ranged from 5–8 days and for the first 24 hours was in an ICU (26). The reasons for this were several-fold. Early in our experience, the ICU was preferred for postoperative monitoring and 1:1 care between the nurse and the patient, think that this would ensure the best outcomes. The problem with this approach was that most patients in the ICU were sick and required close monitoring; whereas, our DIEP flap patients were not sick and did not require aggressive nursing care other than frequent flap monitoring. Thus, specialized nursing wards were implemented to monitor the flaps and care for these patients with the intent to optimally manage our postoperative patients. Our current monitoring protocol is to check Doppler signals every hour for the first 24 hours and then every 4 hours until discharge. The frequency and duration of flap monitoring is surgeon specific.
Also common during the early years of DIEP flap reconstruction, was to infuse Dextran intravenously for 5 days. The rationale for this was that dextran was an inhibitor of platelet aggregation and that it took 5 days for vascular re-endothelization to occur; thus, anastomotic patency would be optimized. This approach was abandoned about 2005 with the realization that the best predictor of anastomotic success was surgical technique. In some patients, intravenous heparin would be infused for anticoagulation purposes. The use of these anticoagulants had to be considered in contrast to the risk of hematoma formation. As our microvascular techniques improved and with the realization that proper technique was the most important factor in demonstrating high success rates for free tissue transfer, most microvascular surgeons abandoned the use of postoperative anticoagulation therapy for anastomotic success but rather began the use of enoxaparin sodium for the prevention of deep venous thrombosis. As a result, most DIEP flap patients will remain in the hospital for 2–3 days with extremely high success rates that in some series exceed 99%.
Another important factor associated with early discharge from the hospital is the implementation of enhanced recovery after surgery (ERAS) protocols (27). During the early days of DIEP flaps, patients were often managed with narcotic analgesia and delayed oral intake that contributed to prolonged hospitalization. ERAS protocols were developed to facilitate the recovery process, reduce narcotic use and promote earlier discharge from the hospital. These protocols are initiated prior to, during and after surgery for maximal effect (27). Preoperative factors include the administration of gabapentin, celecoxib and acetaminophen as well as allowing patients to drink 12 ounces of an electrolyte rich carbohydrate beverage. Intraoperative factors include the use of regional field blocks using local analgesic such as liposomal bupivacaine or Marcaine. Intravenous steroid and Ondansetron as well as a scopolamine patch are useful to prevent postoperative nausea. Postoperative factors include early ambulation and early oral intake as well as administration of non-narcotic analgesics as needed. All of these factors have demonstrated success in allowing patients to be discharged earlier.
Another aspect of postoperative care is the monitoring techniques used to assess flap perfusion. Over the years, there have been various devices used that include the hand-held Doppler, the implantable Doppler and near infrared spectroscopy (14). Interestingly, we have come full circle in this category. From 1995–2010, the hand-held Doppler was used exclusively; however, with the newer technologies, implantable Dopplers and near infrared spectroscopy was used but for only a short time. At present, the hand-held Doppler is used intraoperatively and postoperatively and has demonstrated high reliability and success (28).
Conclusions
The DIEP flap has become a common operation throughout the world and is clearly the most common autologous option provided to women following mastectomy. It has provided the majority of women with excellent outcomes with high patient satisfaction. Operative times have been significantly reduced, hospitalization time has decreased, and surgical outcomes have improved since its inception in 1994. Success rates are now 98–99% for most surgeons performing this operation and hospitalizations now range from 2–3 days for most patients. ERAS protocols have facilitated the postoperative recovery and had a significant positive effect on reducing narcotic consumption. The strengths of this paper include a comprehensive review of advancements associated with the DIEP flap. A potential weakness is that all points of view and perspectives may not have been adequately reviewed and addressed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ketan M. Patel and Ara A. Salibian) for the series “Advances in Microsurgical Breast Reconstruction” published in Gland Surgery. The article has undergone external peer review.
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-636/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-636/coif). The series “Advances in Microsurgical Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. MYN is a consultant for Allergan/AbbVie (Irvine, CA) and Stryker (Kalamazoo, MI). The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this clinical practice review and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Nahabedian MY, Schwartz J. Autologous breast reconstruction following mastectomy. Handchir Mikrochir Plast Chir 2008;40:248-54. [Crossref] [PubMed]
- Nahabedian MY. Achieving ideal breast aesthetics with autologous reconstruction. Gland Surg 2015;4:134-44. [PubMed]
- Nahabedian MY. Achieving ideal donor site aesthetics with autologous breast reconstruction. Gland Surg 2015;4:145-53. [PubMed]
- Nahabedian MY. Factors to consider in breast reconstruction. Womens Health (Lond) 2015;11:325-42. [Crossref] [PubMed]
- ASPS procedural statistics. accessed October 10, 2022. Available online: www.ASPS.org
- Nahabedian MY, Patel K. Autologous flap breast reconstruction: Surgical algorithm and patient selection. J Surg Oncol 2016;113:865-74. [Crossref] [PubMed]
- Nahabedian MY, Momen B, Galdino G, et al. Breast Reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg 2002;110:466-75; discussion 476-7. [Crossref] [PubMed]
- Haddock NT, Kayfan S, Pezeshk RA, et al. Co-surgeons in breast reconstructive microsurgery: What do they bring to the table? Microsurgery 2018;38:14-20. [Crossref] [PubMed]
- Haddock NT, Teotia SS. Efficient DIEP Flap: Bilateral Breast Reconstruction in Less Than Four Hours. Plast Reconstr Surg Glob Open 2021;9:e3801. [Crossref] [PubMed]
- Haddock NT, Teotia SS. Deconstructing the Reconstruction: Evaluation of Process and Efficiency in Deep Inferior Epigastric Perforator Flaps. Plast Reconstr Surg 2020;145:717e-24e. [Crossref] [PubMed]
- Colakoglu S, Tebockhorst S, Freedman J, et al. CT angiography prior to DIEP flap breast reconstruction: a randomized controlled trial. J Plast Reconstr Aesthet Surg 2022;75:45-51. [Crossref] [PubMed]
- Nahabedian MY. Overview of perforator imaging and flap perfusion technologies. Clin Plast Surg 2011;38:165-74. [Crossref] [PubMed]
- Nahabedian MY, Momen B, Manson PN. Factors associated with anastomotic failure after microvascular reconstruction of the breast. Plast Reconstr Surg 2004;114:74-82. [Crossref] [PubMed]
- Rao SS, Parikh PM, Goldstein JA, et al. Unilateral failures in bilateral microvascular breast reconstruction. Plast Reconstr Surg 2010;126:17-25. [Crossref] [PubMed]
- Fitzgerald O'Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg 2016;5:88-92. [PubMed]
- Lorenzetti F, Kuokkanen H, von Smitten K, et al. Intraoperative evaluation of blood flow in the internal mammary or thoracodorsal artery as a recipient vessel for a free TRAM flap. Ann Plast Surg 2001;46:590-3. [Crossref] [PubMed]
- Newman MI, Samson MC. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg 2009;25:21-6. [Crossref] [PubMed]
- Varela R, Casado-Sanchez C, Zarbakhsh S, et al. Outcomes of DIEP Flap and Fluorescent Angiography: A Randomized Controlled Clinical Trial. Plast Reconstr Surg 2020;145:1-10. [Crossref] [PubMed]
- Yoo A, Palines PA, Mayo JL, et al. The Impact of Indocyanine Green Angiography on Fat Necrosis in Deep Inferior Epigastric Perforator Flap Breast Reconstruction. Ann Plast Surg 2022;88:415-9. [Crossref] [PubMed]
- Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: is there a difference? Plast Reconstr Surg 2005;115:436-44. [Crossref] [PubMed]
- Bhullar H, Hunter-Smith DJ, Rozen WM. Fat Necrosis After DIEP Flap Breast Reconstruction: A Review of Perfusion-Related Causes. Aesthetic Plast Surg 2020;44:1454-61. [Crossref] [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Winters H, Tielemans HJP, Hummelink S, et al. DIEP flap breast reconstruction combined with vascularized lymph node transfer for patients with breast cancer-related lymphedema. Eur J Surg Oncol 2022;48:1718-22. [Crossref] [PubMed]
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [Crossref] [PubMed]
- Haddock NT, Garza R, Boyle CE, et al. Observations from Implementation of the ERAS Protocol after DIEP Flap Breast Reconstruction. J Reconstr Microsurg 2022;38:506-10. [Crossref] [PubMed]
- Nahabedian MY, Patel KM. Maximizing the use of the handheld Doppler in autologous breast reconstruction. Clin Plast Surg 2011;38:213-8. [Crossref] [PubMed]



