
Association between the systemic immune-inflammation index and the efficacy of neoadjuvant chemotherapy, prognosis in HER2 positive breast cancer—a retrospective cohort study
Highlight box
Key findings
• The systemic immune-inflammation index (SII) was associated with the prognosis and efficacy of neoadjuvant chemotherapy in human epidermal growth factor receptor 2 (HER2) positive breast cancer patients.
What is known and what is new?
• An increased SII level is associated with a poor prognosis in patients with malignant tumors.
• This study sought to explore the relationship between the SII and neoadjuvant chemotherapy efficacy in HER2 positive breast cancer patients.
What is the implication, and what should change now?
• The SII could be used to predict the prognosis and efficacy of neoadjuvant chemotherapy in HER2 positive breast cancer patients. For patients with an increased SII before neoadjuvant chemotherapy, intensive treatment should be adopted, which may improve the prognosis of patients.
Introduction
Breast cancer is one of the most common malignant tumors in women, and its incidence is increasing year by year (1). According to recent cancer statistics, breast cancer has surpassed lung cancer as the most commonly diagnosed malignant tumor (1). Thus, the prevention of breast cancer needs to be prioritized. A medical and health system with an early detection and early diagnosis mode needs to be established so patients can receive early treatment.
Many studies have explored the factors related to the treatment and prognosis of breast cancer patients (2-5), and the prognosis of such patients needs to be improved. Scientists found that the overexpression of human epidermal growth factor receptor 2 (HER2) was directly related to the growth, invasion and prognosis of tumors (6). About 10–34% of invasive breast cancer patients have HER2 overexpression or HER2 gene amplification (6). HER2 molecules activate mitogen-activated protein kinase and other pathways by forming heterodimers with other receptors of the epidermal growth factor family and thus promote the occurrence and progression of tumors (7).
Neoadjuvant therapy has been widely used in clinical practice to treat breast cancer. Before the emergence of neoadjuvant targeted therapy and neoadjuvant endocrine therapy, neoadjuvant therapy mainly referred to neoadjuvant chemotherapy. Neoadjuvant chemotherapy has indeed benefited some patients. Notably, it has enabled some patients who could not receive breast-conserving therapy to undergo conservative treatment and enabled some patients once thought to be inoperable to undergo surgery. The concept of neoadjuvant therapy now extends to neoadjuvant chemotherapy, neoadjuvant targeted therapy, and neoadjuvant endocrine therapy.
The prognosis of patients who achieve a pathological complete response after neoadjuvant chemotherapy is significantly improved, while the prognosis of those who do not achieve a pathological complete response or even have progressive disease is relatively poor (8). Thus, accurate predictions of the efficacy of neoadjuvant chemotherapy before neoadjuvant chemotherapy could provide a theoretical basis for improving the prognosis of patients in the next step. At present, researchers have found that the expression level of related genes and positron emission tomography/computed tomography can be used to predict the efficacy of neoadjuvant chemotherapy in HER2 positive blast cancer patients, but there are some disadvantages such as high price (9-11).
In recent years, the systemic immune-inflammation index (SII) has become a popular indicator in research. An increased SII level indicates an increased level of inflammation and a decreased level of immune function in patients (12). Thus, an increase in the SII level is related to the poor prognosis of patients with malignant tumors. Moreover, one study showed that the SII was related to the long-term prognosis of triple-negative breast cancer patients (13). Studies on other types of breast cancer patients have also confirmed that an increase in the SII level was related to the poor prognosis of breast cancer patients (14-17). In patients receiving neoadjuvant chemotherapy, studies have also found that the SII was related to the overall survival rate and disease-free survival rate (18,19). In triple-negative breast cancer patients receiving neoadjuvant chemotherapy, Chinese scholars have reported that the SII level was related to the efficacy of neoadjuvant chemotherapy (20). Among HER2 positive breast cancer patients, a study has also shown that an increase in the SII level was related to a poor prognosis after adjuvant therapy (21). However, there is still a lack of research on the relationship between the SII and neoadjuvant chemotherapy in HER2 positive breast cancer patients; thus, we designed this study. We present this article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-55/rc).
Methods
General information
Sample size estimation: each variable needs to include at least 10 events, which has been widely accepted (also known as 10 events per variable) (22). The pathological complete response rate of HER2 positive breast cancer was about 60%, so the estimated sample size was more than 150 (23). This was a retrospective cohort study. The data of 172 HER2 positive breast cancer patients admitted to the Nuclear 215 Hospital of Shaanxi Province from January 2015 to January 2017 were retrospectively continuously collected. After neoadjuvant chemotherapy, the patients were divided into the complete response group (n=70) and the non-complete response group (n=102) based on whether or not they had achieved a pathological complete response (Figure 1).
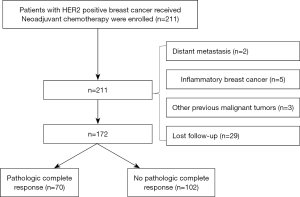
To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have HER2 positive (no special type) of invasive breast cancer with estrogen receptor and progesterone receptor negativity; (II) be a female aged 18–75 years; (III) have a primary tumor diameter >2 cm or axillary lymph node metastasis; (IV) have a Zubrod-Eastern Cooperative Oncology Group-World Health Organization performance status score of 0–1; (V) have received neoadjuvant chemotherapy combined with targeted therapy at the Nuclear 215 Hospital of Shanxi Nuclear Industry; and (VI) have available follow-up data. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had distant metastasis; (II) had inflammatory breast cancer; (III) had received anti-tumor therapy or radiotherapy for any malignant tumor in the past, excluding cured cervical carcinoma in situ, basal cell carcinoma, squamous cell carcinoma, and other malignant tumors; (IV) had also received a special treatment, such as neoadjuvant endocrine therapy; (V) had pregnancy-associated breast cancer; and/or (VI) had organ dysfunction in important organs, such as the heart, lung, liver and kidney.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Nuclear 215 Hospital of Shaanxi Province (No. 20220078). The requirement of individual consent for this retrospective analysis was waived.
Treatment
All the patients were treated with 4 cycles of Epirubicin + Cyclophosphamide and the following 4 cycles of Paclitaxel + Trastuzumab with 21 days as a cycle. The first 4 cycles used doxorubicin combined with cyclophosphamide, and the last 4 cycles used paclitaxel and trastuzumab. After the neoadjuvant chemotherapy, the patients received surgical treatment, and continued to use trastuzumab for 1 year after the surgery.
Follow-up
The follow-up was carried out in the form of outpatient appointments combined with telephone calls after the surgery. Breast ultrasound, chest computed tomography (CT), and abdominal CT were performed at least once every 6 months, and dynamic contrast-enhanced magnetic resonance imaging of the breast was performed once every 2 years. For patients in whom recurrence or metastasis was suspected, a bone scan or whole-body positron emission tomography-CT was performed.
Standard definitions
(I) HER2 positivity: before neoadjuvant chemotherapy, an ultrasound-guided biopsy was performed to obtain a breast cancer tissue sample and immunohistochemical staining was performed. According to the staining ratio and staining intensity of the HER2 protein on the cell membrane, the expression level of HER2 was defined as 0, 1+, 2+, 3+, where HER2 0 represents a negative staining result or the presence of staining on <10% of the tumor cells; HER2 1+ indicates that the light and barely visible cell staining intensity was displayed on >10% of the tumor cells; HER2 2+ indicates that the weak to moderate staining intensity of the intact cell membrane was displayed on 10–30% of the tumor cells; HER2 3+ indicates that the strong staining intensity of the intact membrane was displayed on >30% of the tumor cells. If HER2 3+ was defined as positive, for patients with HER2 2+, a fluorescence in situ hybridization test was performed. HER2 gene amplification was considered HER2 positive, otherwise it was considered negative. (II) Pathological complete response: a patient achieved a pathological complete response if no evidence of invasive breast cancer was found in the primary breast and lymph nodes in the metastatic region, or only carcinoma in situ was found. (III) SII: the following formula was used to calculate the SII: SII = (neutrophils percentage × platelets count)/lymphocytes percentage.
Study variables
The study variables were as follows: age at diagnosis, body mass index, lesion site, family history, diabetes, hypertension, hyperlipidemia, proliferation index of tumor cells (Ki-67), the SII, tumor size, lymph node metastasis, histological grade, number of lesions, recurrence and metastasis after surgery.
Statistical analysis
All the statistical analyses were performed using Statistical Package for the Social Science 26.0 (IBM, Chicago, USA). A 2-tailed P value <0.05 was considered statistically significant. In this study, the SII and other measurement data conformed to a normal distribution, and are expressed as the mean ± standard deviation, and the independent sample t-test was used to analyze differences between the two groups. The postoperative recurrence and other counting data are expressed as the number (percentage), and the chi-square test was used to analyze differences between the two groups. A receiver operating characteristic curve was used to analyze the value of the SII in predicting a pathological complete response after neoadjuvant chemotherapy. A multivariate logistic regression analysis was conducted to explore the risk factors of postoperative recurrence or metastasis in breast cancer. When the area under the curve is 0.7–0.9, it indicates that it has certain clinical significance. When the area under the curve is greater than 0.9, it indicates that the prediction accuracy is high.
Results
Comparison of the clinical characteristics of the two groups
The SII level of the complete response group was significantly lower than that of the non-complete response group (587.43±175.97 vs. 821.82±231.58; P=0.000). Additionally, the tumor size (4.68±1.53 vs. 5.45±1.59 cm; P=0.002), rate of lymph node metastasis (20.00% vs. 50.00%; P=0.000), 5-year recurrence rate (12.86% vs. 27.45%; P=0.022), and 5-year metastasis rate (5.71% vs. 15.69%; P=0.045) (Table 1) were significantly decreased in the complete response group compared to the non-complete response group.
Table 1
| Variables | Complete response group (n=70) | Non-complete response group (n=102) | t/χ2 value | P value |
|---|---|---|---|---|
| Age at diagnosis (years) | 46.33±16.83 | 47.30±14.88 | 0.400 | 0.689 |
| Body mass index (kg/m2) | 23.59±3.70 | 24.37±3.94 | 1.323 | 0.188 |
| Site of lesion | 2.574 | 0.109 | ||
| Left | 27 (38.57) | 52 (50.98) | ||
| Right | 43 (61.43) | 50 (49.02) | ||
| Family history | 2 (2.86) | 3 (2.94) | 0.001 | 0.974 |
| Diabetes | 4 (5.71) | 7 (6.86) | 0.091 | 0.762 |
| Hypertension | 6 (8.57) | 7 (6.86) | 0.173 | 0.677 |
| Hyperlipidemia | 5 (7.14) | 9 (8.82) | 0.157 | 0.692 |
| Surgery | 0.062 | 0.803 | ||
| Breast-conserving surgery | 12 (17.14) | 19 (18.63) | ||
| Mastectomy | 58 (82.86) | 83 (81.37) | ||
| Ki-67 (%) | 41.31±22.83 | 44.18±24.36 | 0.777 | 0.439 |
| SII | 587.43±175.97 | 821.82±231.58 | 7.165 | 0.000 |
| Tumor size (cm) | 4.68±1.53 | 5.45±1.59 | 3.195 | 0.002 |
| Lymph node metastasis | 14 (20.00) | 51 (50.00) | 15.892 | 0.000 |
| Histological grade | 0.059 | 0.809 | ||
| Grade II | 33 (47.14) | 50 (49.02) | ||
| Grade III | 37 (52.86) | 52 (50.98) | ||
| Number of lesions | 0.157 | 0.692 | ||
| Single lesion | 65 (92.86) | 93 (91.18) | ||
| Multiple lesions | 5 (7.14) | 9 (8.82) | ||
| 5-year recurrence rate | 9 (12.86) | 28 (27.45) | 5.236 | 0.022 |
| 5-year metastasis rate | 4 (5.71) | 16 (15.69) | 4.017 | 0.045 |
Data are expressed as mean ± standard deviation or n (%). SII, systemic immune-inflammation index.
Value of the SII and tumor size in predicting no pathological complete response in the HER2 positive breast cancer patients after neoadjuvant chemotherapy
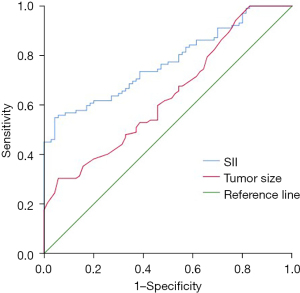
The SII level had some value in predicting which HER2 positive breast cancer patients would fail to achieve a pathological complete response after neoadjuvant chemotherapy. The area under the curve (AUC) was 0.773 [95% confidence interval (CI): 0.705–0.804; P=0.000]. The best diagnostic threshold was 755.10, the sensitivity was 0.618, and the specificity was 0.800. The tumor size had some value in predicting which HER2 positive breast cancer patients would fail to achieve a pathological complete response after neoadjuvant chemotherapy. The AUC was 0.639 (95% CI: 0.557–0.722; P=0.002). The best diagnostic threshold was 4.95 cm, the sensitivity was 0.598, and the specificity was 0.543 (Figure 2).
Related factors of the HER2 positive breast cancer patients who achieved a pathological complete response after neoadjuvant chemotherapy
A SII >755.10 and lymph node metastasis were adverse factors for indicating which HER2 positive breast cancer patients would achieve a pathological complete response after neoadjuvant chemotherapy (P<0.05) (Table 2).
Table 2
| Variables | B | Standard error | Wald | P value | RR (95% CI) |
|---|---|---|---|---|---|
| A SII >755.10 | –1.762 | 0.375 | 22.098 | 0.000 | 0.172 (0.082–0.358) |
| Tumor size >4.95 cm | –0.464 | 0.355 | 1.702 | 0.192 | 0.629 (0.313–1.262) |
| Lymph node metastasis | –1.222 | 0.387 | 9.997 | 0.002 | 0.295 (0.138–0.628) |
| Constant | 5.918 | 1.067 | 30.785 | 0.000 | 371.704 |
HER2, human epidermal growth factor receptor 2; SII, systemic immune-inflammation index; RR, relative risk; CI, confidence interval.
Correlation between the SII and postoperative recurrence within 5 years of surgery
The SII level had some value in predicting recurrence in 5 years after surgery, and had an AUC of 0.828 (95% CI: 0.757–0.900; P=0.000) (Figure 3). A SII >755.10 was a risk factor for recurrence within 5 years of surgery [P=0.001, relative risk (RR): 4.945 (95% CI: 1.949–12.544)] (Table 3).
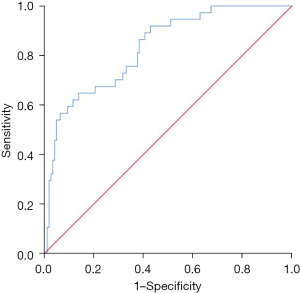
Table 3
| Variables | B | Standard error | Wald | P value | RR (95% CI) |
|---|---|---|---|---|---|
| A SII >755.10 | 1.598 | 0.475 | 11.326 | 0.001 | 4.945 (1.949–12.544) |
| Tumor size >4.95 cm | –0.018 | 0.461 | 0.001 | 0.970 | 0.983 (0.398–2.427) |
| Lymph node metastasis | 2.668 | 0.508 | 27.617 | 0.000 | 14.404 (5.326–38.954) |
| Constant | –4.811 | 1.136 | 17.926 | 0.000 | 0.008 |
SII, systemic immune-inflammation index; RR, relative risk; CI, confidence interval.
Correlation between the SII and postoperative metastasis within 5 years of surgery
The SII level had some value in predicting metastasis within 5 years of surgery, and had an AUC of 0.837 (95% CI: 0.756–0.917; P=0.000) (Figure 4). A SII >755.10 was a risk factor for metastasis within 5 years of surgery (P=0.014, RR: 4.553 (95% CI: 1.362–15.220)] (Table 4).
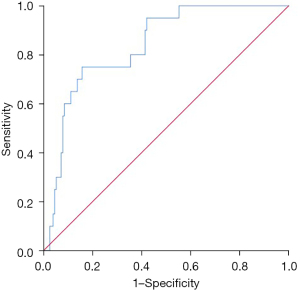
Table 4
| Variables | B | Standard error | Wald | P value | RR (95% CI) |
|---|---|---|---|---|---|
| A SII >755.10 | 1.516 | 0.616 | 6.062 | 0.014 | 4.553 (1.362–15.220) |
| Tumor size >4.95 cm | 0.283 | 0.558 | 0.258 | 0.612 | 1.328 (0.445–3.964) |
| Lymph node metastasis | 2.826 | 0.775 | 13.306 | 0.000 | 16.884 (3.698–77.091) |
| Constant | –4.296 | 1.400 | 9.414 | 0.002 | 0.014 |
SII, systemic immune-inflammation index; RR, relative risk; CI, confidence interval.
Discussion
Neoadjuvant chemotherapy plays an important role in the diagnosis and treatment of breast cancer. The efficacy of neoadjuvant chemotherapy is directly related to the overall survival rate of patients after surgery. Thus, it is of great significance to predict the efficacy of neoadjuvant chemotherapy.
This study explored the relationship between the SII level and neoadjuvant chemotherapy efficacy in HER2 positive breast cancer patients. We found that the SII level in patients with a pathological complete response after neoadjuvant chemotherapy was significantly reduced, and the SII level had a high value in predicting a pathological complete response after neoadjuvant chemotherapy, The multivariate regression analysis showed that a SII >755.10 was an adverse factor for HER2 positive breast cancer patients to achieve a pathological complete response after neoadjuvant chemotherapy (P<0.05). After 5 years of follow-up, we also found that an increase in the SII was a risk factor for recurrence and metastasis.
As stated above, the SII is calculated as follow: SII = (neutrophils percentage × platelets count)/lymphocytes percentage. An increase in the SII level indicates an increase in the level of neutrophils and platelets, and a decrease in the level of lymphocytes. Neutrophils represent the level of inflammation in patients. An increased level of inflammation is conducive to angiogenesis in the tumor microenvironment and the immune escape of tumors, which in turn lead to the rapid proliferation and metastasis of breast cancer cells (24). Tumor cells release active factors that promote the synthesis of platelets, accelerate the synthesis of platelets, and lead to a high platelet count, which is also a common laboratory manifestation of cancer (25).
Additionally, the compensatory enhancement of bone marrow hematopoietic function leads to the acceleration of platelet synthesis, which leads to the increase of platelets. Platelets promote the proliferation and metastasis of tumor cells and form a malignant cycle with the invasion and the metastasis of tumors (25). At present, research in breast cancer patients has confirmed that an increase in platelets is related to poor patient prognosis (26-28).
Lymphocytes are the main immune mechanism by which the body kills tumor cells. When the level of lymphocytes decreases, the ability of the body to kill malignant tumors decreases, which in turn leads to the immune escape of tumor cells, and ultimately a poor prognosis (29-31).
Based on the above-mentioned evidence, scholars synthesized these 3 indicators to form a new indicator; that is, the SII. At present, research has confirmed the role of the SII level in breast cancer patients, which is related to patient prognosis, and also has a clear correlation with the efficacy of neoadjuvant chemotherapy for triple-negative breast cancer patients (15-21). However, the relationship between the SII level and the efficacy of neoadjuvant chemotherapy in patients with HER2 positive breast cancer remains unclear. This study confirmed that the SII level affected the efficacy of neoadjuvant chemotherapy in patients with HER2 positive breast cancer.
Limitations
This study was a retrospective clinical study, which failed to explore the molecular mechanism by which the SII level affects neoadjuvant chemotherapy efficacy in HER2 positive breast cancer patients.
Conclusions
The SII was associated with the prognosis and efficacy of neoadjuvant chemotherapy in HER2 positive breast cancer patients. For patients with an increased SII level before neoadjuvant chemotherapy, the treatment plan should be strengthened, which may in turn improve their prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-55/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-55/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-55/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Nuclear 215 Hospital of Shaanxi Province (No. 20220078). The requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- Qiu Y, Chen H, Dai Y, et al. Nontherapeutic Risk Factors of Different Grouped Stage IIIC Breast Cancer Patients' Mortality: A Study of the US Surveillance, Epidemiology, and End Results Database. Breast J 2022;2022:6705052. [Crossref] [PubMed]
- He XC, Chen HY, Qiu Y, et al. Associations of iron status with breast cancer risk factors in adult women: Findings from National Health and Nutrition Examination Survey 2017-2018. J Trace Elem Med Biol 2021;68:126867. [Crossref] [PubMed]
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022;72:524-41. [Crossref] [PubMed]
- Xia X, Hu T, He X, et al. Neddylation of HER2 Inhibits its Protein Degradation and promotes Breast Cancer Progression. Int J Biol Sci 2023;19:377-92. [Crossref] [PubMed]
- Yau C, Osdoit M, van der Noordaa M, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol 2022;23:149-60. [Crossref] [PubMed]
- Wang Q, Wang X, Yang Y. Advances in neoadjuvant therapy for HER2-positive breast cancers: a narrative review. Gland Surg 2022;11:1415-23. [Crossref] [PubMed]
- Yuan JQ, Xiao Z, Wang SM, et al. The prognostic effect of HER2 heterogeneity and YAP1 expression in HER2 positive breast cancer patients: a retrospective study. Gland Surg 2022;11:451-65. [Crossref] [PubMed]
- Koolen BB, Pengel KE, Wesseling J, et al. FDG PET/CT during neoadjuvant chemotherapy may predict response in ER-positive/HER2-negative and triple negative, but not in HER2-positive breast cancer. Breast 2013;22:691-7. [Crossref] [PubMed]
- Xu T, Zhang SM, Wu HM, et al. Prognostic significance of prognostic nutritional index and systemic immune-inflammation index in patients after curative breast cancer resection: a retrospective cohort study. BMC Cancer 2022;22:1128. [Crossref] [PubMed]
- Li W, Ma G, Deng Y, et al. Systemic Immune-Inflammation Index Is a Prognostic Factor for Breast Cancer Patients After Curative Resection. Front Oncol 2021;11:570208. [Crossref] [PubMed]
- Zhang Y, Sun Y, Zhang Q. Prognostic value of the systemic immune-inflammation index in patients with breast cancer: a meta-analysis. Cancer Cell Int 2020;20:224. [Crossref] [PubMed]
- Hua X, Long ZQ, Zhang YL, et al. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Breast Cancer: A Propensity Score-Matching Study. Front Oncol 2020;10:580. [Crossref] [PubMed]
- Sun Y, Li W, Li AJ, et al. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manag Res 2019;11:3153-62. [Crossref] [PubMed]
- Liu J, Shi Z, Bai Y, et al. Prognostic significance of systemic immune-inflammation index in triple-negative breast cancer. Cancer Manag Res 2019;11:4471-80. [Crossref] [PubMed]
- Chen L, Kong X, Wang Z, et al. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med 2020;24:2993-3021. [Crossref] [PubMed]
- Jiang C, Lu Y, Zhang S, et al. Systemic immune-inflammation index is superior to neutrophil to lymphocyte ratio in prognostic assessment of breast cancer patients undergoing neoadjuvant chemotherapy. Biomed Res Int 2020;2020:7961568. [Crossref] [PubMed]
- Pang J, Wang S, Liao L, et al. Association between systemic immune-inflammation index and neoadjuvant chemotherapy efficacy as well as prognosis in triple-negative breast cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2021;46:958-65. [PubMed]
- Jiang L, Fang J, Ding J. High Systemic Immune-Inflammation Index Predicts Poor Survival in Patients with Human Epidermal Growth Factor Receptor-2 Positive Breast Cancer Receiving Adjuvant Trastuzumab. Cancer Manag Res 2020;12:475-84. [Crossref] [PubMed]
- Broll S, Glaser S, Kreienbrock L. Calculating sample size bounds for logistic regression. Prev Vet Med 2002;54:105-11. [Crossref] [PubMed]
- Kurozumi S, Inoue K, Takei H, et al. ER, PgR, Ki67, p27(Kip1), and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer 2015;15:622. [Crossref] [PubMed]
- Zou H, Liu SH, Yang R, et al. Combination of Neutrophil-to-Lymphocyte Ratio and Red Cell Distribution Width With Serum Tumor Markers for the Differential Diagnosis of Breast Cancer and its Association With Pathological Features and Molecular Types. Clin Breast Cancer 2022;22:e526-35. [Crossref] [PubMed]
- Garmi N, Nasrallah S, Baram Y, et al. Platelets and Breast Cancer. Isr Med Assoc J 2020;22:613-7. [PubMed]
- Koyama Y, Kawai S, Uenaka N, et al. Absolute Lymphocyte Count, Platelet-to-Lymphocyte Ratio, and Overall Survival in Eribulin-treated HER2-negative Metastatic Breast Cancer Patients. Cancer Diagn Progn 2021;1:435-41. [Crossref] [PubMed]
- Morkavuk ŞB, Kocaöz S, Korukluoğlu B. Diagnostic value of Platelet/lymphocyte Ratio (PLR) for predicting sentinel axillary lymph node positivity in early-stage breast cancer compared with ultrasonography. Int J Clin Pract 2021;75:e14939. [Crossref] [PubMed]
- Al Jarroudi O, El Bairi K, Abda N, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of outcomes in inflammatory breast cancer. Biomark Med 2021;15:1289-98. [Crossref] [PubMed]
- Wang Y, Zong B, Yu Y, et al. Ki67 Index Changes and Tumor-Infiltrating Lymphocyte Levels Impact the Prognosis of Triple-Negative Breast Cancer Patients With Residual Disease After Neoadjuvant Chemotherapy. Front Oncol 2021;11:668610. [Crossref] [PubMed]
- Wortman JC, He TF, Solomon S, et al. Spatial distribution of B cells and lymphocyte clusters as a predictor of triple-negative breast cancer outcome. NPJ Breast Cancer 2021;7:84. [Crossref] [PubMed]
- Uchida S, Kojima T, Sugino T. Clinicopathological Features, Tumor Mutational Burden, and Tumour-Infiltrating Lymphocyte Interplay in ERBB2-Mutated Breast Cancer: In Silico Analysis. Pathol Oncol Res 2021;27:633243. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


