
Retrospective study of characteristics and hyperthermia intraperitoneal perfusion in mucinous borderline ovarian tumor and mucinous ovarian carcinoma
Highlight box
Key findings
• Overall survival was not statistically different between fertility-sparing surgery and non-fertility-sparing surgery in patients with mucinous borderline ovarian tumor; hyperthermic intraperitoneal perfusion chemotherapy can improve the survival of patients with advanced mucinous ovarian cancer.
What is known and what is new?
• Hyperthermic intraperitoneal perfusion chemotherapy and optimal cytoreductive surgery are considered feasible therapy options for advanced ovarian epithelial carcinoma associated with peritoneal cancer.
• This manuscript recorded a different hyperthermic intraperitoneal perfusion chemotherapy protocol and the value of hyperthermic intraperitoneal perfusion therapy in patients with mucinous borderline ovarian tumor or mucinous ovarian cancer.
What is the implication, and what should change now?
• Our study suggested that the use of hyperthermic intraperitoneal perfusion therapy may improve the prognosis of mucinous borderline ovarian tumor and mucinous ovarian cancer. Future research should explore the feasibility of thermal perfusion therapy inpatients with mucinous borderline ovarian tumor. Multi-center, randomized controlled studies of the use of hyperthermic intraperitoneal perfusion chemotherapy in mucinous tumors should be performed.
Introduction
Epithelial ovarian tumor accounts for about 65–75% of all types of ovarian tumors (1). Mucinous ovarian tumor accounts for 15% of all epithelial ovarian tumors including mucinous benign ovarian tumor, mucinous borderline (low malignant potential) ovarian tumor (MBOT), and mucinous ovarian cancer (MOC) (2). Mucinous borderline ovarian tumors, which are epithelial ovarian tumors somewhere between benign and malignant, refer to epithelial cells with certain malignant characteristics and non-destructive interstitial infiltration, but can be accompanied by extra-ovarian lesions, and some of these patients die due to recurrence and progression of the disease. In addition, about 13% of MBOT patients can progress to invasive carcinoma within 10 years (3). Previous research found that the incidence of MBOT developing into invasive carcinoma reached 13% over 10 years, which suggested that mucinous borderline tumors posed some risks, even after therapy. it is usually diagnosed in middle-aged women with large pelvic masses and the median age of patients with MBOT is 10–20 years younger than the patients with MOC, approximately 90% of MBOT are stageI at diagnosis. There are concerns regarding the risk of intraoperative cyst rupture, whereby mucus enters the abdominal and pelvic cavity, which can induce peritoneal hyperplasia and metaplasia into mucusproducing epithelium, forming peritoneal myxoma, which accounts for 2–5% of ovarian mucinous tumors; the 5-year survival rate is about 45–54%, and the 10-year survival rate is about 18% (4). These patients are in the fertile period, the accuracy of the frozen section is crucial because it will change the extent of surgery (4).
Primary MOC accounts for 3–10% of all epithelial ovarian cancer (5). MOC usually presents as a huge pelvic mass which can be symptomatic or asymptomatic at diagnosis (2). There are concerns regarding the risk of intraoperative cyst rupture, whereby mucus enters the abdominal and pelvic cavity, which can induce peritoneal hyperplasia and metaplasia into mucus-producing epithelium, forming peritoneal myxoma, which accounts for 2–5% of ovarian mucinous tumors; the 5-year survival rate is about 45–54%, and the 10-year survival rate is about 18% (6). Previous study has shown that women with MOC have a worse prognosis in contrast to women with other subtypes of epithelial ovarian cancers after being matched for stage and other factors (7). At present, complete surgical resection is still the gold-standard therapy for all MOC (8). In addition to surgery, advanced-stage ovarian carcinomas are often treated with adjuvant carboplatin-based chemotherapy (8); however, these regimes were developed largely against the more common high-grade serous ovarian carcinoma and have lower efficacy in treating MOC (8).
Because of the differences in clinical behavior and prognosis of MBOT and MOC, more effective therapeutics are needed.Researchers from different countries have been seeking to uncover more effective means to manage patients with mucinous carcinoma. As a novel therapy, hyperthermic intraperitoneal perfusion chemotherapy (HIPEC) has been used for ruptured MBOT, MOC, and peritoneal pseudomyxomas (9). Some studies have suggested that HIPEC and optimal cytoreductive surgery are considered feasible therapy options for advanced ovarian epithelial carcinoma associated with peritoneal cancer (10,11). Peritoneal Surface Oncology Group International (PSOGI) reported the results of a study evaluating HIPEC in patients with rare types of ovarian malignancies including mucous carcinoma, malignant ovarian sex cord stromal tumor, malignant germ cell tumor, and small cell carcinoma; the procedure of HIPEC is feasible in peritoneal ovarian malignant tumor therapy and could improve the prognosis of patients, but their results need further evaluation (12).
Therefore, in this study, we retrospectively analyzed the clinicopathological features of patients with MBOT or MOC. Furthermore, we analyzed the application of a different HIPE procedure in MBOT and MOC in our single institution. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-45/rc).
Methods
Patient population
This was a single-center, retrospective cohort study. This retrospective study was approved by the Ethics Committee of Henan Provincial People’s Hospital (No. 2021-153-01), and each patient signed a written informed consent prior to the therapy procedures. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The clinical of 240 patients that were diagnosed with mucinous borderline tumors or mucinous ovarian cancer through the hospital pathology registry system from January 2009 to December 2019 data were collected and reviewed retrospectively. Among these patients, 176 had MBOT, and 64 were diagnosed with MOC.
Distribution criteria: inclusion criteria: (I) signed informed consent from the ethics committee of the hospital; (II) clinical data, surgical data and pathological data were complete; (III) the postoperative pathological results confirmed the diagnosis of ovarian mucinous tumors according to the 2014 WHO classification, including borderline and malignant. Exclusion criteria: (I) combined with other benign and malignant female reproductive system diseases; (II) with digestive tract tumors or benign and malignant tumors of other systems; (III) with severe brain, heart, lung, liver, kidney, and surgical diseases; (IV) excluding the metastatic mucinous tumors; (V) patients did not receive follow-up or were not satisfied with follow-up; (VI) uncomplete medical record data; (VII) patients without paraffin histopathologic results.
Diagnostic criteria
Surgical and pathological staging of the patients were based on the International Federation of Gynecology and Obstetrics (FIGO Staging System, 2018), and the pathological diagnosis was confirmed by 2 pathologists after surgery.
Surgical procedures and hyperthermic intraperitoneal perfusion
The staging surgeries for patients with MBOT were classified into the following categories: (I) fertility sparing surgery (preservation of the uterus, 1 ovary, or cystectomy) for premenopausal women who desired pregnancy; (II) radical staging surgery was done for women who did not desire future pregnancy. In our study, the patients with MBOT were divided into 3 groups: complete staging group, incomplete staging group, and unstaged procedure group. Staging surgery was considered complete if peritoneal washing, omentectomy, appendectomy, and peritoneal biopsies were performed. If any of these 4 staging procedures was excluded, the staging was considered incomplete. If only ovarian cystectomy or oophorectomy was performed, it was considered unstaged (13). All Patients with MOC underwent primary debulking surgery and received six cycles of systemic chemotherapy (Paclitaxel 175 mg/m2 + oxaliplatin 130 mg/m2). Surgical results were divided into 2 categories: (I) optimal cytoreductive surgery whereby 1 or more macroscopic tumors measuring the diameter less than 1 cm was not resected; (II) incomplete cytoreductive surgery that 1 or more residual lesions measuring larger than 1 cm in diameter remained after surgery (14).
Of these patients, a proportion underwent dense HIPE using a custom-developed high-precision body cavity HIPE system (BR-TRG-II; Bright Medical Technology Co. Ltd., Guangzhou, China) by closed procedures. Due to low economic status, and any other personal or consideration, the other proportion of patients refused HIPE after operative procedure. The procedure of dense HIPE has been reported in a previous report (14). The procedure comprised 4 perfusion catheters being separately placed into the hemidiaphragm and pelvis, and an intra-abdominal temperature of 42–43 ℃ was maintained by circulation of the heated saline. Perfusion was conducted in equal amounts during each 60-minute HIPEC session with a 450–600 mL/min flow rate which was adjusted such that the entire abdomen was exposed to the perfusate; the perfusion liquid was drained before the next session. The 4 catheters were removed after the final HIPE session (14). Some of the patients with MOC underwent dense HIPE with cisplatin (dose 60 mg/m2, each session) and some of the patients with MBOT were underwent 3 sessions of dense HIPE without cisplatin. The HIPE procedures were performed in the general ward.
Follow-up
The following information was reviewed and collected: age, pelvic ultrasound, computed tomography (CT) scan or magnetic resonance imaging (MRI), and serum biomarkers [carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199), human epididymis protein 4 (HE4)], histopathological subtypes, frozen section (FS) diagnosis, surgical procedure, postoperative management, tumor recurrence (imaging diagnosis) and death time, adverse events of HIPE.
Patient follow-up included symptoms, physical examination, and measurement of the CA125, CA199, and HE4 levels were measured every 3 months for 2 years and every 6 months up to 5 years. Either CT or MRI was performed at least 1, 3, 6, 12, 18, and 24 months after the chemotherapy. Clinical, radiologic oprogression after the initial induction therapy and time to normalization was identified as PFS. OS was defined as the time from the date of therapy to the date of either death from any cause or final follow-up. Safety assessment was performed according to the US national cancer institute’s patient-reported outcomes version of the common terminology criteria for Adverse Events (PRO-CTCAE). The follow-up time was until 31 October 2021.
Statistical analyses
All data were analyzed with the statistical software SPSS 21.0 (IBM Corp., Armonk, NY, USA). Descriptive data analysis was conducted as median ± SD for continuous variables and χ2 tests for categorical variables. Survival analysis was performed with the Kaplan-Meier method; Cox proportional hazards model with a 95% Wald confidence interval (95% CI) was used to analyze the hazard ratios (HRs). A two-sided P value of <0.05 was considered statistically significant.
Results
Patient demographics and clinical characteristics
The characteristics of the patients are summarized in Table 1. A total of 176 patients were diagnosed with MBOT, among whom the mean age was 34 years (range, 13–81 years), and 64 patients with MOC were identified, among whom the mean age was 59 years (range, 28–69 years); the patients with MOC had significant older age and higher rate of advanced staging tumor than those with MBOT. Among the 176 patients with MBOT, 97 underwent radical surgery (55.1%), and 79 underwent conservative surgery (44.9%). A total of 154 patients with MBOT had complete staging procedures (87.5%), there were 158 with stage I disease (89.8%), 3 with stage II (1.7%) disease, and 15 with stage III (8.5%) disease, 43 patients who underwent HIPE and 113 patients who did not undergo HIPE, and only 6 patients had lymph node metastases. Among the 64 patients with MOC, only 6 underwent fertility sparing surgery (9.4%), 38 patients with HIPEC (59.4%) and 26 patients without HIPEC (40.6%); all patients with MOC underwent 6 cycles of systemic chemotherapy (paclitaxel 175 mg/m2 + oxaliplatin 130 mg/m2), and 17 patients had lymph node metastases.
Table 1
| Characteristics | Patients with MBOT (n=176) | Patients with MOC (n=64) |
|---|---|---|
| Age (years), mean [range] | 34 [13–81] | 59 [28–69] |
| Rupture, n (%) | ||
| Yes | 64 (36.4) | 25 (39.1) |
| No | 112 (63.6) | 39 (60.1) |
| Surgery, n (%) | ||
| Fertility sparing surgery | 79 (44.9) | 6 (9.4) |
| Radical surgery | 97 (55.1) | 58 (90.6) |
| Incomplete staging surgery | 57 (32.4) | 6 (9.4) |
| Hyperthermic intraperitoneal therapy, n (%) | ||
| Yes | 59 (33.5) | 38 (59.4) |
| No | 117 (66.5) | 26 (40.6) |
| Position, n (%) | ||
| Left | 69 (39.2) | 31 (48.4) |
| Right | 78 (44.3) | 29 (45.3) |
| Both | 29 (16.5) | 4 (6.3) |
| Stage, n (%) | ||
| I | 158 (89.8) | 24 (37.5) |
| II | 3 (1.7) | 4 (6.3) |
| III | 15 (8.5) | 32 (50.0) |
| IV | 0 | 4 (6.3) |
| Lymph node metastasis, n (%) | ||
| Yes | 6 (3.4) | 17 (26.6) |
| No | 170 (96.6) | 47 (73.4) |
MBOT, mucinous borderline ovarian tumor; MOC, mucinous ovarian cancer.
Serum biomarkers in MBOT and MOC
Among the 240 patients in our institution, 147 underwent preoperative serum CA125 testing, and 59 (40.1%) had an elevated level (normal ≤35 U/mL), the level was greater than 100 U/mL in 31 patients; 132 patients received preoperative serum CA199 testing, and 53 patients (40.2%) had a greater than 35 U/mL, the level was more than 100 U/mL in 36 cases; preoperative HE4 levels of 71 patients were obtained, and only 4 patients had a level greater than the reference value. Figure 1A-1C depicts the levels of serum biomarkers in MBOT and MOC.

Clinicopathological features and prognosis for patients
According to the postoperative pathological features, 124 of the 176 patients (70.5%) with MBOT had gastrointestinal MBOT, and there were 52 cases (29.5%) of endocervical MBOT, 10 patients also had peritoneal pseudomyxoma, 3 patients had pelvic endometriosis, and 3 also had mature teratoma. Of these patients with MOC, 7 had borderline tumors or intraepithelial carcinoma. All 176 patients with MBOT underwent frozen-section examination. The frozen-section diagnosis was consistent with paraffin-embedded diagnosis in 109 patients (61.9%); 62 had a low-accuracy diagnosis (frozen-section diagnosis of benign), whereas over-diagnosis (frozen-section diagnosis suggested malignancy) was found in 5 cases. Among the patients with MBOT, the median follow-up was 73 and 32 months in patients with HIPE and without HIPE, respectively. A total of 18 cases relapsed, there were 5 cases of peritoneal myxoma, 3 cases of stromal microinvasion, and 1 case of intraepithelial carcinoma. Of those 79 patients who underwent fertility preserving surgery, 9 had recurrent disease and chose to repeat surgery, among whom, 7 had mucinous borderline tumors, 2 had benign mucinous cystadenoma, and none progressed to carcinoma. The median time from surgery to the initial recurrence was 27 months, and all relapses were within 5 years. Only 1 recurrence was recorded in the patients with HIPE. The total recurrence rate of 176 cases of mucinous borderline tumors was 10.23%. These characteristics of the 176 patients are shown in Table 2.
Table 2
| Variables | Total | Recurrence | P value |
|---|---|---|---|
| Histology | 0.862 | ||
| Gastrointestinal | 124 | 13 | |
| Seromucinous | 52 | 5 | |
| Primary surgery | 0.243 | ||
| Cystectomy | 22 | 5 | |
| Complete staging surgery of fertility sparing | 57 | 7 | |
| Radical | 97 | 6 | |
| Stage | 0.002 | ||
| I | 158 | 12 | |
| III | 15 | 6 | |
| Hyperthermic intraperitoneal therapy | 0.078 | ||
| No | 133 | 17 | |
| Yes | 43 | 1 |
Of 64 patients who were diagnosed with MOC, the median follow-up was 69 months (27–92 months), 37 recurrences were identified, including 2 cases of IC stage, 1 case of stage II, 30 cases of stage III, and 4 cases of stage IV. The characteristics of the patients are summarized in Table 3.
Table 3
| Variables | With HIPEC (n=38) | Without HIPEC (N=26) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 56.38±12.726 | 53.42±11.456 | 0.345 |
| Histology | 0.876 | ||
| Gastrointestinal | 27 | 18 | |
| Seromucinous | 11 | 8 | |
| Stage | 0.847 | ||
| I | 15 | 9 | |
| II | 2 | 2 | |
| III and IV | 21 | 15 | |
| Cyto-reductive surgery (<1 cm) | 0.976 | ||
| Yes | 35 | 24 | |
| No | 3 | 2 |
MOC, mucinous ovarian cancer; HIPEC, hyperthermic intraperitoneal perfusion chemotherapy; SD, standard deviation.
Moreover, of 36 patients who were diagnosed with advanced stage MOC (staging III and IV), 21 received 3 sessions of HIPEC after the primary cytoreductive surgery (PDS) as the PDS plus HIPEC group, and 15 underwent the primary cytoreductive surgery without HIPEC as the PDS group.
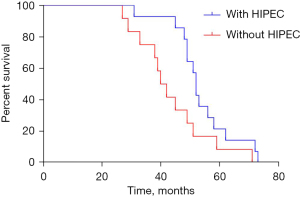
In PDS with HIPEC group, the median progression-free survival (PFS) was 27 (95% CI: 24.819–29.181) months, compared to 19 (95% CI: 19.062–24.935) months in the PDS group (P=0.001, Figure 2). The median OS was 53 (95% CI: 48.486–57.514) months in the PDS with HIPEC group and 42 (95% CI: 37.198–46.802) months in the PDS group (P=0.010, Figure 3). Significant differences were observed between the groups in terms of both the median PFS (HR =0.273; 95% CI: 0.122–0.609; P=0.000) and median OS (HR =0.361; 95% CI: 0.160–0.814; P=0.001).

Adverse events of HIPEC
In the PDS with HIPEC group of patients with advanced MOC, no adverse events were recorded in 11 (52.4%) of 21 patients, and 3 (14.3%) of 21 patients experienced grade III adverse events. Meanwhile, in the PDS group, 9 (60%) of 15 patients experienced no adverse events, and 2 (13.3%) of the 15 patients were reported to have experienced grade III adverse events. These common adverse events observed in this study are summarized in Tables 4,5. The most common grade I–II adverse event was electrolyte disturbance, and the most common grade III adverse event was ileus, which is similar to previous research (13). All HIPEC procedures were performed in the general ward and not in the intensive care unit. There was no death with the first 30 days postoperatively and there were no severe fatal complications.
Table 4
| Adverse events | Grade I or II, n (%) | Grade III, n (%) |
|---|---|---|
| Hematology | ||
| Leucocytopenia | 3 (14.3) | 0 |
| Thrombocytopenia | 0 | 0 |
| Anemia | 6 (28.6) | 0 |
| Electrolyte disturbance* | 7 (33.3) | 1 (4.8) |
| Thromboembolism | 2 (9.5) | 0 |
| Nausea | 3 (14.3) | 0 |
| Emesis | 4 (19.1) | 0 |
| Nephrotoxicity | 0 | 0 |
| Abdominal pain | 5 (23.8) | 1 (4.8) |
| Ileus | 1 (4.8) | 3 (14.3) |
| Anastomotic hemorrhage | 0 | 0 |
| Infection | 3 (14.3) | 1 (4.8) |
*, electrolyte disturbances included hyponatremia, hypochloridemia, hypokalemia, hypocalcemia, hypomagnesemia, and hypophosphatemia. MOC, mucinous ovarian cancer; HIPEC, hyperthermic intraperitoneal perfusion chemotherapy.
Table 5
| Adverse events | Grade I or II, n (%) | Grade III or IV, n (%) |
|---|---|---|
| Hematology | ||
| Leucocytopenia | 0 | 0 |
| Thrombocytopenia | 0 | 0 |
| Anemia | 4 (26.7) | 0 |
| Electrolyte disturbance* | 4 (26.7) | 0 |
| Thromboembolism | 1 (6.7) | 0 |
| Nausea | 3 (20.0) | 0 |
| Emesis | 0 | 0 |
| Nephrotoxicity | 0 | 0 |
| Abdominal pain | 3 (20.0) | 0 |
| Ileus | 1 (6.7) | 2 (13.6) |
| Anastomotic hemorrhage | 0 | 0 |
| Infection | 3 (20.0) | 0 |
*, electrolyte disturbances included hyponatremia, hypochloridemia, hypokalemia, hypocalcemia, hypomagnesemia, and hypophosphatemia. Infection included lung infection, urinary tract infection, urethral infection, stomal site infection, sepsis, bacteremia, abdominal infection. MOC, mucinous ovarian cancer; HIPEC, hyperthermic intraperitoneal perfusion chemotherapy.
Discussion
The World Health Organization (WHO) has classified borderline ovarian tumors (BOT) as a stand-alone group of ovarian epithelial tumors since the 1970; BOT are not rare clinical tumors, which represent 10–20% of ovarian tumors (15). Due to the characteristics of recurrence, implantation, metastasis, and malignant transformation, the WHO has defined it as low-grade ovarian malignant tumor in recent years (16). MBOT account for 30–50% of BOT (13). An identified consequence of epidemiological studies is that MBOT are usually diagnosed in women during their reproductive period (17). Previous reports have shown that MBOT is diagnosed at an average age of 34–49 years (18-21). In a Chinese study, Sun et al. reported that the median age of patients with MBOT was 40 years (21); the present study collected a larger number of MBOT cases, and the median age was found to be 34 years with the age interval of 13 to 81 years. The findings of our study are similar to ranges reported in previous reports, the difference in median age may be attributed to intrinsic factors such as race, region and sample size. Fortunately, BOT presents as a disease more frequently limited to the ovaries compared with invasive carcinoma; du Bois et al. reported a systematic review of 6,362 cases among which 78.9% of the patients with BOT were diagnosed at FIGO stage I (22), FIGO stages II–III are rarely seen at the time of diagnosis, and no cases were diagnosed FIGO stage IV (17). In a multicenter study, 90% of patients were diagnosed as FIGO stage I at the time of surgery for MBOT, equally, in this study, FIGO I disease was diagnosed at a very high rate (89.8%), whereas FIGO II and FIGO III stage diseases were diagnosed at a low rate (10.2%), these results also reveal that conservative management with preservation of fertility may be feasible, and this objective and reliable information should be given to patients who wish to preserve fertility. Contrastingly, in serous epithelial ovarian cancer, more than 70% of cases were diagnosed at an advanced stage, and in our study, more than 40% of cases with MOC were diagnosed at an early stage (FIGO I and II). Ovarian mucinous tumors often present as pelvic masses, with or without symptoms. Previous study has reported an algorithm based on the size of tumor which can be used to determine the type of mucinous ovarian tumors, but some data do not support this algorithm (23). In addition to physical examination and history, tumor markers are also important to predict the nature of mucinous ovarian tumors. As we know, serum CA125 assay has sensitivity for epithelial ovarian cancer and has low sensitivity in the early stages (24), but there are limited data in the MBOT and MOC, Dochez et al. reported evidence supporting that serum CA199 has a stronger association with mucinous tumors than other cell types of epithelial ovarian tumors (23). HE4 has been found to be a reliable biological marker for epithelial ovarian carcinoma (23) but there are no data for their associations with MBOT and MOC. In the previous literature, tumor markers were reported to help to improve the accuracy of the FS for mucinous ovarian tumors (25). In this report, we evaluated these 3 serum biomarkers (CA125, CA199, and HE4) in MBOT and MOC. Some 40.1% of patients with MBOT had elevated serum levels of CA125 and 40.2% of these patients had a similar increase of CA199, but only 5.6% of these patients had a slight increase in HE4 level. Elevated CA125 levels are also reported in other conditions such as endometriosis, pregnancy, menstruation, and inflammatory diseases of the peritoneum, therefore, serum CA199 combined with CA125 are useful markers to distinguish benign from borderline or malignant mucinous tumors; however, HE4 is not suitable for borderline mucinous tumors. On the contrary, levels of CA125, CA199, and HE4 were elevated remarkably in malignant mucinous ovarian cancer, further, the levels of these 3 serum biomarkers were found to increase significantly. To date, several meta-analyses or systematic reviews have shown that the combination of CA125 and HE4 is an effective diagnostic tool in ovarian cancer (23,26). According to the data of our study, the association of CA125, CA199, and HE4 may be used to distinguish MBOT from MOC.
As patients commonly experience MBOT in the fertile period, an accurate FS diagnosis is crucial because it greatly impacts the surgical approach and may be reduce the number of re-operations. Most studies have reported that accuracy rates in MBOT are lower than in other histological types. Due to their large size and high heterogeneity, under-diagnosis or over-diagnosis are more common in MBOT (18,27). The findings of the current study revealed that the rates of under-diagnosis and over-diagnosis of MBOT by FS biopsy were 35.2% and 2.8%; the accuracy rate of FS examination diagnosis was 61.9%. In the present study, we included a relatively large number of patients with MBOT, and our results are similar to this of previous studies (7,18,27). This low accuracy may be due to the following reasons: first, because the tumor diameter for MBOT is larger than that of other histological types, it is difficult to accurately estimate the entire tumor pathology by FS for each centimeter of the neoplasm in a limited time; second, technical parameters such as sampling doctor and freezing process could influence the FS accuracy, third, MBOT are composed of so many complex components that benign, borderline, or invasive lesions can be presented in the same specimen; fourth, if extensive sampling is not possible, the pathologist may choose safety for diagnosis in the cases of doubt; finally, specialized gynecological pathologists are also important. Therefore, the under-diagnosis of MBOT poses a more urgent problem as the under-diagnosis of MBOT and invasive mucinous carcinomas frequently lead to revision surgeries.
In previous studies, more than 90% of patients were diagnosed with FIGO I disease at the time of surgery for MBOT, similarly, in this report, 89% of patients with MBOT were also diagnosed with stage I disease, these results reveal that the incidence of extra-gonadal MBOT (stage ≥ FIGO II) is rare. At the same time, we found that there was no statistical difference in the recurrence rate between incomplete staging surgery and complete staging surgery. Ovarian cystectomy may lead to an increased postoperative recurrence rate. Additionally, in the patients with staging surgery, only 3.9% (6/154) had lymphatic metastases. Other study reported that lymphatic metastases accounted for 21% of cases, but no change in PFS and OS (28), and other data have suggested that the rate of recurrence is higher after ovarian cystectomy. Gungorduk et al. reported that the prognosis of patients with MBOT is excellent and that detailed surgical staging does not have an impact on survival rates (13). Therefore, women with FIGO I–II MBOT could be considered for surgery without lymphadenectomy, appendices, and omentectomy. The expansion of the surgical scope instead increases the incidence of perioperative complications. This conclusion still needs to be verified by further prospective studies.
According to the WHO 2014 histological classification, MBOT is divided into 2 major pathological subtypes: gastrointestinal subtype and serousmucinous subtypes. Sun et al. found that gastrointestinal MBOT account for 73.7% of all MBOT cases and some of the subtypes are combined with intraepithelial carcinoma or microinvasion or peritoneal pseudomyxoma; serousmucinous subtypes account for 26.3% of MBOT and some of cases were associated with ipsilateral ovarian or pelvic endometriosis (21). In our study, 176 MBOT cases were analyzed retrospectively, of which gastrointestinal MBOT accounted for 70.5% (124/176) and serousmucinous MBOT accounted for 29.5% (52/176). There was no significant difference (P=0.862) in the postoperative recurrence rate of these 2 subtypes. Most mucinous tumors stem from gastrointestinal tract, the appendix, and it is important to exclude metastatic adenocarcinoma in the diagnosis of mucinous ovarian borderline tumors. Carr reported that the ovary is only rarely the source of pseudomyxoma, and that lesions which would previously have been called “borderline mucinous tumors of the ovary” are typically metastatic from the appendix (29).
Numerous studies confirm the association of several pathological and clinical characteristics with an increased risk of MBOT relapse, including invasive peritoneal implants (30), advanced stage, cyst rupture (31), stromal microinvasion (32), and elevated baseline CA125 (33). Our data showed that in the 18 recurrence cases, only 1 had intraepithelial carcinoma, 3 had micro-infiltrative and 5 had peritoneal pseudomyxoma. The vast majority of MBOT are large, we suspect that some of these cases involve missed detection of invasive carcinoma or cyst rupture. According to an expert consensus of China that recommended HIPE to be used for ruptured MBOT, we have performed HIPE for some patients with MBOT in recent years. However, due to the short follow-up time, only 1 patient relapsed. There was no significant difference between the HIPE group and the non-hyperthermic intraperitoneal perfusion therapy group. Prospective studies are needed to explore the feasibility of HIPE therapy in patients with MBOT.
Historically, all epithelial ovarian cancers have been treated in a similar fashion, however, mucinous ovarian carcinoma represents less than 5% of all epithelial ovarian malignancies, with a specific clinical presentation and biological behavioral pattern (34,35). Compared with all other epithelial ovarian cancers, it appeared that outcomes of MBOT were worse and age of patients were younger. Previous clinical trials including the Gynecologic Oncology Group (GOG) trials 111, 132, 182, and GOG172 involved patients with all types of ovarian tumors, and mucinous ovarian tumors comprised a low proportion (1.6–3.4%). In recent years, more and more studies have revealed that standard therapy with HIPEC significantly enhances the survival of ovarian cancer patients, especially in randomized, controlled trials (36). HIPEC may result in better prognoses after complete cytoreduction for mucinous ovarian cancer patients. In a multicenter, open-label, phase 3 trial which included 245 ovarian cancer patients randomly assigned to 2 groups according to whether they received HIPEC after underging interval cytoreductive surgery, significant improvement was observed in OS: 33.9 versus 47.5 months in the surgery alone and surgery with HIPEC groups, respectively (36). Likewise, our results showed a median OS of 53 months and a median PFS of 27 for the HIPEC group versus 42 and 19 months, respectively, for patients without HIPEC, which reveals that HIPEC improved the median PFS by 8 months and the median OS by 11 months; complete cytoreduction with HIPEC combined paclitaxel and oxaliplatin significantly improved survival rates of patients with advanced mucinous ovarian cancer. Moreover, the incidence of adverse events was similar and no grade IV adverse events were recorded in these 2 groups. Importantly, we did not observe any grade III renal failure, which is the same as previous reports indicating that HIPEC can be tolerated by Chinese patients (14,35). It is worth noting that the HIPEC procedure was performed in the operating room or the intensive care unit in other studies to increasing patient safety. However, HIPEC was performed in the general ward without any associated adverse events and was well tolerated by the included patients.
Limitations
The main limitation of this study is the short follow-up period for the application of HIPE in patients with MBOT, and the follow-up needs to be continued in order to count the apparent recurrence rate and further carry out to explore the feasibility of HIPE in patients with MBOT. Furthermore, our study was performed retrospectively in a single center, in the future, multi-center, randomized controlled studies should be performed.
Conclusions
According to this retrospective analysis, patients with MBOT are of a younger age, MBOT is diagnosed at an early stage and has a good prognosis, and incompletely staged surgery does not increase the recurrence rate for MBOT. A substantial number of MBOT were misdiagnosed by frozen section diagnosis, the association of CA125, CA199, and HE4 may be used to assist in accurate diagnosis. The feasibility and necessity of HIPE in MBOT need to be further explored. Ovarian mucinous carcinoma is relatively common in the early stage, and hyperthermic intraperitoneal perfusion chemotherapy can improve the survival of patients with advanced mucinous carcinoma.
Acknowledgments
Funding: This research was supported by the Henan Provincial Medical Science and Technology Public Relations Program Project (Nos. LHG20200024 and 2018020423).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-45/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-45/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-45/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was ethically inspected and approved by the Ethics Committee of Henan Provincial People’s Hospital (No. 2021-153-01) and written informed consent for this study was signed by all patients involved and recorded in their medical records.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Lertkhachonsuk AA, Buranawongtrakoon S, Lekskul N, et al. Serum CA19-9, CA-125 and CEA as tumor markers for mucinous ovarian tumors. J Obstet Gynaecol Res 2020;46:2287-91. [Crossref] [PubMed]
- Feng Z, Chen T, Li X, et al. Analysis of the clinicopathological characteristics of ovarian borderline mucinous tumors. Hebei Pharma 2020;42:3221-5.
- Shim SH, Kim SN, Jung PS, et al. Impact of surgical staging on prognosis in patients with borderline ovarian tumours: A meta-analysis. Eur J Cancer 2016;54:84-95. [Crossref] [PubMed]
- Gaballa K, Abdelkhalek M, Fathi A, et al. Management of borderline ovarian tumors: A tertiary referral center experience in Egypt. Front Surg 2022;9:962820. [Crossref] [PubMed]
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovfarian cancer: a review. Cancer Biol Med 2017;14:9-32. [Crossref] [PubMed]
- Zhang Y, Li C, Luo S, et al. Retrospective Study of the Epidemiology, Pathology, and Therapeutic Management in Patients With Mucinous Ovarian Tumors. Technol Cancer Res Treat 2020;19:1533033820946423. [Crossref] [PubMed]
- Craig O, Salazar C, Gorringe KL. Options for the Treatment of Mucinous Ovarian Carcinoma. Curr Treat Options Oncol 2021;22:114. [Crossref] [PubMed]
- Valenzuela CD, Levine EA, Mangieri CW, et al. Repeat Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Cancers with Peritoneal Metastasis: A 30-year Institutional Experience. Ann Surg Oncol 2022;29:3436-45. [Crossref] [PubMed]
- Mishra M, Singh N, Ghatage P. Past, Present, and Future of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Ovarian Cancer. Cureus 2021;13:e15563. [Crossref] [PubMed]
- Lei Z, Wang Y, Wang J, et al. Evaluation of Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Stage III Epithelial Ovarian Cancer. JAMA Netw Open 2020;3:e2013940. [Crossref] [PubMed]
- Goéré D, Passot G, Gelli M, et al. Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: results from a worldwide analysis issue of the Peritoneal Surface Oncology Group International (PSOGI). Int J Hyperthermia 2017;33:520-7. [Crossref] [PubMed]
- Gungorduk K, Asicioglu O, Braicu EI, et al. The Impact of Surgical Staging on the Prognosis of Mucinous Borderline Tumors of the Ovaries: A Multicenter Study. Anticancer Res 2017;37:5609-16. [PubMed]
- He X, Wei L, Li R, et al. Dense hyperthermic intraperitoneal chemotherapy with cisplatin in patients with stage III serous epithelial ovarian cancer: a retrospective study. BMC Cancer 2021;21:738. [Crossref] [PubMed]
- Meinhold-Heerlein I, Fotopoulou C, Harter P, et al. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet 2016;293:695-700. [Crossref] [PubMed]
- Palomba S, Zupi E, Russo T, et al. Comparison of two fertility-sparing approaches for bilateral borderline ovarian tumours: a randomized controlled study. Hum Reprod 2007;22:578-85. [Crossref] [PubMed]
- Gershenson DM. Management of borderline ovarian tumours. Best Pract Res Clin Obstet Gynaecol 2017;41:49-59. [Crossref] [PubMed]
- Karataşlı V, Can B, Çakır İ, et al. Effect of tumor size on the accuracy of frozen section in the evaluation of mucinous borderline ovarian tumors. J Gynecol Obstet Hum Reprod 2020;49:101765. [Crossref] [PubMed]
- Park JY, Lee SH, Kim KR, et al. Accuracy of frozen section diagnosis and factors associated with final pathological diagnosis upgrade of mucinous ovarian tumors. J Gynecol Oncol 2019;30:e95. [Crossref] [PubMed]
- Cömert DK, Üreyen I, Karalok A, et al. Mucinous borderline ovarian tumors: Analysis of 75 patients from a single center. J Turk Ger Gynecol Assoc 2016;17:96-100. [Crossref] [PubMed]
- Sun L, Li N, Song Y, et al. Clinicopathologic Features and Risk Factors for Recurrence of Mucinous Borderline Ovarian Tumors: A Retrospective Study With Follow-up of More Than 10 Years. Int J Gynecol Cancer 2018;28:1643-9. [Crossref] [PubMed]
- du Bois A, Trillsch F, Mahner S, et al. Management of borderline ovarian tumors. Ann Oncol 2016;27:i20-2. [Crossref] [PubMed]
- Dochez V, Caillon H, Vaucel E, et al. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res 2019;12:28. [Crossref] [PubMed]
- Zhang M, Cheng S, Jin Y, et al. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer 2021;1875:188503. [Crossref] [PubMed]
- Zhang W, Jia S, Xiang Y, et al. Factors associated with misdiagnosis of frozen section of mucinous borderline ovarian tumor. J Int Med Res 2019;47:96-104. [Crossref] [PubMed]
- Wang J, Gao J, Yao H, et al. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta-analysis. Tumour Biol 2014;35:6127-38. [Crossref] [PubMed]
- Huang Z, Li L, Li C, et al. Diagnostic accuracy of frozen section analysis of borderline ovarian tumors: a meta-analysis with emphasis on misdiagnosis factors. J Cancer 2018;9:2817-24. [Crossref] [PubMed]
- Camatte S, Morice P, Thoury A, et al. Impact of surgical staging in patients with macroscopic "stage I" ovarian borderline tumours: analysis of a continuous series of 101 cases. Eur J Cancer 2004;40:1842-9. [Crossref] [PubMed]
- Carr NJ. Current concepts in pseudomyxoma peritonei. Ann Pathol 2014;34:9-13. [Crossref] [PubMed]
- Song T, Lee DH, Jung YW, et al. Elevated Preoperative CA125 or CA19-9 in Borderline Ovarian Tumors: Could It Be Suggestive of Advanced Stage or a Poor Prognosis? Gynecol Obstet Invest 2018;83:45-51. [Crossref] [PubMed]
- Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001;357:176-82. [Crossref] [PubMed]
- McKenney JK, Balzer BL, Longacre TA. Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): a reevaluation of the concept of stromal microinvasion. Am J Surg Pathol 2006;30:1209-21. [Crossref] [PubMed]
- Babaier A, Ghatage P. Mucinous Cancer of the Ovary: Overview and Current Status. Diagnostics (Basel) 2020;10:52. [Crossref] [PubMed]
- Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep 2014;16:389. [Crossref] [PubMed]
- Hess V, A'Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol 2004;22:1040-4. [Crossref] [PubMed]
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med 2018;378:230-40. [Crossref] [PubMed]
(English Language Editor: J. Jones)


