
Clinical experience of the Magseed® magnetic marker to localize non-palpable breast lesions: a cohort study of 100 consecutive cases
Highlight box
Key findings
• The Magseed® magnetic marker combined with the Sentimag® probe is an effective method to localize non-palpable breast lesions.
What is known and what is new?
• Several reports have demonstrated the feasibility and safety of this technique compared to the wire-guided localization (WGL) technique.
• We demonstrated our clinical experience on 100 consecutive patients with whom we used the Magseed® for identification of subclinical breast lesions and is effective when extended microcalcification clusters are present, or in targeting multiple tumors within the same breast.
What is the implication, and what should change now?
• Further studies are needed to validate this technique with all practitioners and also to prove its efficacy of other long-term applications for patients who require neoadjuvant treatment.
IntroductionOther Section
In recent years, the implementation of mammography screening programs as well as new medical imaging technologies such as tomosynthesis or artificial intelligence and women’s increased awareness of breast cancer, have resulted in an increase in the detection rate of non-palpable lesions (1-5). These tumors are too small in size or located too deep in the breast parenchyma to be detected during clinical examination. Consequently, techniques for pre-operative identification of non-palpable cancerous lesions during breast conservation surgery have been developed. The success of breast conservation surgery requires effective communication between the radiologist and the surgeon, and precise guidance by the radiologist to allow the surgeon to perform complete removal of the lesion with healthy resection margins, while striving to preserve as much as possible the breast’s aesthetic curve. However, this also requires operating schedule management and the practitioner availability. There are many guide techniques available. The standard localization method used is wire-guided localization (WGL), in which wire hook is placed into the tumor with ultrasound or stereotactic guidance (6,7). However, this technique has several disadvantages. One of the most important being that the guide wire has to be placed within a few hours of surgery to minimize the risk of dislocation. This requires interdependence of radiology and surgery schedules, reducing operating room efficiency. Furthermore, wire guides cause discomfort for patients and migration or transection can occur (8). The WGL may also limit alternatives for placement and the distance between the entry site and the tip of wire may be greater, thus leading to extensive dissection and affecting potential aesthetic outcome. Consequently, alternative guide methods were developed such as radioactive seed localization (RSL), non-radioactive radar localization, radio frequency identification and magnetic seed localization (9). In this study, we evaluated the Magseed® magnetic marker (Endomagnetics, Cambridge, UK). The Magseed® marker is a non-radioactive paramagnetic seed that can be inserted under ultrasound or with stereotactic guidance. During surgery, the seed is detected using a magnetic detection probe (Sentimag®, Sysmex, GmBH, Hamburg, Germany). Several reports have demonstrated the feasibility and safety of this technique (10-16). The aim of this study is to report the experience of 100 consecutive cases where the Magseed® marker was used to localize non-palpable breast lesions. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-552/rc).
MethodsOther Section
Patient selection
This retrospective study includes 100 patients who were managed at the Center of Senology Drs Crèvecoeur (a private radiology center) and the CHC MontLegia hospital between May 2019 and April 2021. Patients with non-palpable lesions undergoing breast-conservation surgery were deemed suitable for seed insertion following discussion among the multidisciplinary team. We also included patients with complete or partial response of neoadjuvant chemotherapy (NAC). For these patients, an intramammary clip was placed before their scheduled NAC. The follow-ups during their chemotherapy assessing response were performed by mammography and ultrasound. At the end of treatment, magnetic resonance imaging (MRI) was performed to obtain the precise dimension of the residual tumor, or the marker clip released before NAC in case of complete tumor response, was then marked by the Magseed® marker. Exclusion criteria were pregnancy/lactation or any mental condition with might render the patient incapable of giving written consent and patients who denied to sign the consent form. The study was approved by the CHC MontLegia Ethics Committee (reference No. 20/40/1052) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all participants included.
Description of guide method
We used the Magseed® marker (Endomagnetics, Cambridge, UK), a magnetic seed localization technique. It consists of a 5 mm × 1 mm cylindrical non-radioactive paramagnetic surgical steel marker that can be visualized via mammography or ultrasound. It is supplied in sterile packaging preloaded into an 18-gauge 7-cm steel needle. The marker can be inserted at any time before surgery. In the operating room, the Magseed® marker is detected using the Sentimag® probe which generates an alternating magnetic field, transiently magnetizing the seed and subsequently measuring the magnetic field emitted by the seed.
Technique
Seeds were inserted using an aseptic technique by ultrasound guidance. All seeds were inserted by the same breast radiologist. Xylocaine 2% was used to anesthetize the skin and a small incision was made with a blade to facilitate insertion of the introducer needle. Two mammograms (craniocaudal and mediolateral views) were carried out to confirm the position of the seed at the target site and successful localization. X-ray dimensions of the tumor, as well as the distance between the seed and the tumor were documented. This procedure was performed for solid tumors. In the cases of lesions with microcalcifications, seeds were inserted by ultrasound guidance using the biopsy clip as a target. For some cases, no biopsy clip was present, so the radiologist used the post-biopsy haematoma to insert the Magseed® marker on the ultrasound guidance. If we did not have a biopsy clip or ultrasound image (like hematoma) to visualise the lesion, we took an X-ray with the introducer needle in the breast before dropping the magseed. In case of incorrect positioning, we had the possibility to correct the trajectory. The stereotactic guidance to target residual calcification was not used because the displacement or migration of the clip during decompression was frequently observed. Finally, if the patient had two non-palpable lesions or extensive microcalfication clusters, two Magseed® markers were inserted.
Surgical excision
Once in the operating room, the surgeon used the Sentimag® system’s probe to locate the Magseed® marker with precision. The surgeon was guided by the two mammograms, which allowed placement with the Sentimag® probe in the correct place on the breast’s skin, and with the Sentimag® units that give the numerical counter along with its audio tone. This enabled the surgeon to gauge the distance between the seed and the probe. As soon as the lesion was localized (maximum magnetic signal), the surgeon made an incision and inserted the probe to confirm the seed’s position. Once excised, the surgeon then used the probe to confirm that the seed was included in the specimen. For this study, surgical excision was carried out by two breast surgeons trained in the technique.
Specimen radiography was carried out for each patient with microcalcifications to provide reassurance that all the lesions and seeds had been excised. The examination of the specimen radiography was performed by the breast radiologist who inserted the Magseed® marker.
Pathological examination
The pathologist analyzed the specimen, both the dimensions of the specimen and the tumor were documented. Macroscopic extemporaneous examination was performed for all solid tumors for detection of the seed and margins evaluation. Then the tissue was formalin-fixed, paraffin-embedded and entirely sectioned at 4-µm intervals for the definitive histopathological examination.
Postoperative histology was discussed at a multidisciplinary breast meeting and re-excision or mastectomy was proposed to patients if positive margins has been observed.
Analysis
Data were extracted from the electronic medical record after written consent of patient. Data included patient age, radiological features, placement/localization of seed, identification/retrieval of seed, surgical features and pathologic results. Percentages for categorical variables, mean and median were calculated as appropriate.
ResultsOther Section
Patient characteristics and Magseed® marker insertion
In the study, one hundred patients were included; their baseline characteristics are shown in Table 1. A total of 111 Magseed® markers were placed into the breasts of 100 patients using ultrasound guidance. No infection, hematoma or migration problems were observed. All Magseed® markers were retrieved, as confirmed by specimen radiography or by the pathologist during extemporaneous analysis. The average age of women was 63 years. Magseed® markers were placed a median of 4 days before surgery. Majority of the Magseed® markers (88.3%) were inserted successfully into the target lesion.
Table 1
| Patients characteristics | Number |
|---|---|
| No. of women | 100 |
| Age (years), mean [range], median | 63 [32–78], 66 |
| No. of seeds | 111 |
| Number of days between Magseed® marker placement and surgery, mean [range], median | 5.3 [0–42], 4 |
| Placement success, n (%) | |
| ≤1 mm | 98 (88.3) |
| 2–5 mm | 7 (6.3) |
| 6–10 mm | 6 (5.4) |
Figure 1 shows an example of a Magseed® marker placed very precisely next to the biopsy clip. This clip was placed during macrobiopsy via stereotactic biopsy of the microcalcification clusters. Radiography of the surgical specimen (Figure 1C) confirmed the resection of the microcalcifications, the Magseed® marker and the biopsy clip.
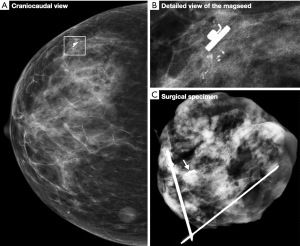
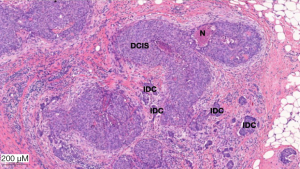
Histological examination of the surgical specimen demonstrated the presence of microcalcification and neoplastic cells corresponding to ductal carcinoma in situ (Figure 2). Actin combined with P63 showed the persistence of myoepithelial layer cells around all glandular structures and the absence of invasive tumor processes.

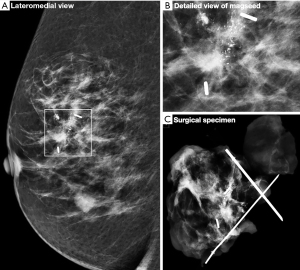
Although the majority of cases involved identification of the lesion with one Magseed® marker some cases required the use of two markers. For 89 women, one Magseed® marker was inserted into their lesion which corresponded to either a mass or microcalcifications. When two lesions were diagnosed in the same breast, they were located with two Magseed® markers. In cases with extended microcalcification clusters, two seeds were deployed to bracket the lesion (Figure 3). Histological analysis of the tumorectomy specimen shown in Figure 3 demonstrate the presence of invasive process (Figure 4).
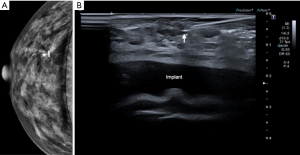
The Magseed® technique also allows the identification of non-palpable lesions such as a radial scar (Figure 5). The case illustrated in Figure 5 corresponds to a patient with breast implants. The radiologist successfully placed the seed despite the presence of breast implants.
Surgical excision
All Magseed® markers were located with a Sentimag® probe by the surgeon. Intraoperative identification and excision of the localized lesion was successful in 100% of patients. In patients where Magseed® markers was used for bracketing microcalcifications, the median size of the lesion was 27.5 mm and the mean size was 36.3 mm. Regarding patients with two solid tumors, the median size between Magseed® markers was 25 mm and the mean size 24.6 mm. For two patients, two Magseed® markers were placed at a distance of less than 10 mm and this small distance complicated their detection. The diagnosis for these patients was made by a radiologist from outside our team and described a bi-focal lesion. As a precaution, the surgeon preferred to use two Magseed® markers. In one case, we also performed a skin surface marking.
Pathological examination
During examination of the specimens by the pathologist, identification of the Magseed® marker was not always straightforward. Whenever necessary, an X-ray of the surgical specimen was performed. From the time of their placement to the time of surgery, no migration of the Magseed® marker in the breast was observed. Majority of resected tumors were solid tumors. From a histological point of view, 57.6% of the patients had invasive ductal carcinoma, 16.2% ductal carcinoma in situ, and 9.0% invasive lobular carcinoma (Table 2). The radiological dimensions for the masses were on average of 7.0 mm and for microcalcifications 24.6 mm. The majority of the microcalcification clusters were small. One patient presented with an extensive microcalcification clusters corresponding of 100 mm. A good correlation between radiological-pathological dimensions were observed for solid tumors. Regarding dimensions of microcalcifications, sizes of lesions described by the pathologist and the radiologist were different. Radiological dimensions were performed before the diagnostic biopsy leading to reduction in lesion extent.
Table 2
| Target lesion characteristics | Number (%) | Radiological dimensions (mm) | Pathological dimensions (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | |||
| Type of lesion | ||||||||
| Solid tumor | 89 (80.2) | 7.0 | 7.5 | 0–40 | 9.5 | 9.0 | 0–50 | |
| Microcalcifications | 22 (19.8) | 24.6 | 20.0 | 3–100 | 19.4 | 15.0 | 3–100 | |
| Specimen volume (cm3) | ||||||||
| Solid tumor | – | – | – | – | 27.6 | 21.0 | 4.7–141.3 | |
| Microcalcifications | – | – | – | – | 59.6 | 26.0 | 5.5–403.0 | |
| Surgical pathology findings | ||||||||
| Invasive ductal carcinoma | 64 (57.6) | – | – | – | – | – | – | |
| Ductal carcinoma in situ | 18 (16.2) | – | – | – | – | – | – | |
| Invasive lobular carcinoma | 10 (9.0) | – | – | – | – | – | – | |
| Response after neoadjuvant chemotherapy | 5 (4.5) | – | – | – | – | – | – | |
| Other invasive tumor | 5 (4.5) | – | – | – | – | – | – | |
| Radial scar | 2 (1.8) | – | – | – | – | – | – | |
| Fibroadenoma | 2 (1.8) | – | – | – | – | – | – | |
| Benign | 3 (2.7) | – | – | – | – | – | – | |
| Lobular carcinoma in situ | 1 (0.9) | – | – | – | – | – | – | |
| No atypical epithelial hyperplasia | 1 (0.9) | – | – | – | – | – | – | |
Complete excisions of the different lesions were performed with disease-free margins of at least 1 mm in 95% of patients. Five patients underwent further surgery for positive margins and pathological finding are described in Table 3. Of these, two patients had a further re-excision margins and three had radical mastectomy. For these five patients, each Magseed® marker was placed in the center of the tumor lesion (≤1 mm). None of the cases were attributed to the localization technique.
Table 3
| Pathological findings for patients with positive margins | Number [%] |
|---|---|
| DCIS | 2 [40] |
| IDC and DCIS | 2 [40] |
| ILC and LCI | 1 [20] |
DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LCI, lobular carcinoma in situ.
DiscussionOther Section
The present study illustrates the use of the Magseed® marker, a promising technique allowing localization of non-palpable breast lesion. Previous reports have demonstrated safety, feasibility and clinical use of magnetic seeds (10-18). We report our experience using the Magseed® magnetic marker and the Sentimag® probe in a Belgian breast unit. Our team had been using the standard WGL technique for intraoperative tumor localization for 30 years. Several disadvantages and limitations linked to the use of WGL, including the risk of migration or the need to place the wire on the same day as surgery, have led to the development of new guide techniques. This study demonstrates that the Magseed® marker offers a convincing clinical alternative to the wire hook. Moreover, this technique allows us greater flexibility in our scheduling, while allocating personnel more effectively and optimizing theater utilization, reducing costs and delays.
Our study describes our experience with the Magseed® marker in a cohort of 100 patients. Implementation of the Magseed® System was straightforward, with a short period of adaptation for the surgeon and the radiologist. Consequently, in a short period, this method has effectively replaced guide wire use. One of the main advantages of the Magseed® marker is that the seed can be placed several days before surgery allowing dissociation of the radiology and surgery schedules. In addition, the Magseed® marker has now been licensed for use over 30 days before the surgery. Timing is undefined provided there is the intention to excise. There were no apparent problems associated with the length time of the Magseed® marker was inserted prior to surgery. In a few cases, the Magseed® marker was placed on the day of surgery. The longest time between placement and removal was 42 days. All the lesions were successfully removed along with the Magseed® marker suggesting no migration of the markers. No other problems were reported. The Magseed® marker is principally used for non-palpable breast lesions but since there is no defined time limit for the removal of the seed, it could be inserted before NAC. In this case, it is of real benefit for the patient who would only need one type of guide system to localize their tumor instead of two. The follow-up of the patient during their chemotherapy to assess response could be carried out solely by mammography and ultrasound. MRI use is not recommended to perform because the Magseed® marker can create a bloom artefact which measures up to 4 cm (19,20). Further studies must be carried out in order to validate the Magseed® marker for tumor localization in patients undergoing NAC.
Our findings demonstrate a relatively low re-excision rate of 5%. This rate is significantly lower than that reported by other studies comparing WGL to the Magseed® marker (19). Zacharioudakis et al. observed no significant difference between the Magseed® cohort (16%) and the WGL cohort (14%) (13). This low re-excision rate observed in our study can be explained in several ways. Firstly, the same radiologist placed all 111 Magseed® markers. Secondly, both surgeons involved in this study were specialized in oncological breast surgery. In addition, for 87% of the patients included in our study, the radiologist carried out the diagnosis, the placement of the Magseed® marker and the radiography of the surgical specimen when necessary. Good preoperative communication between the radiologist and the surgeon is essential. For each patient, the radiologist explained to the surgeon how the Magseed® was inserted (distance between tumor and seed, insertion point, etc.) using mammogram images. Among the patients with positive resection margins, MRIs were carried out for three patients. MRIs did not highlight tumor extension and radiological dimensions were similar to those on mammography and ultrasound. In one patient, the diameter of the tumor was 8 mm, therefore not meeting MRI criteria, and the pathological analysis of the specimen showed a non-calcified ductal carcinoma in situ. With regard to the last patient with positive resection margins, diagnosis was not made by the radiologist who inserted the Magseed® marker. This confirms the influence on patient care if several radiologists are involved. Another difference between the WGL and the Magseed® marker lies in the initial incision. Indeed, surgical approach and incision placement is independent of localization access. This allows a more aesthetic result on the breast’s curve.
We also demonstrated the feasibility of targeting multiple lesions in the same breast. Five patients had two Magseed® markers placed in the same breast to localize two tumors. Two markers were deployed to bracket extended microcalcifications clusters for six patients. There was no difficulty in discriminating between the different sites. For patients with microcalcifications, the distance between Magseed® markers was superior to 20 mm. However, two patients had two lesions less than 1 cm apart. For safety reasons, two Magseed® markers were placed. Despite the complexity in differentiating between the markers, the surgeon proceeded with successful removal of both. Some reports have demonstrated that the Magseed® marker was not detectable if the tumor lesion was deeper than 6 cm (10-22). In our study, one Magseed® was placed at a depth of 3.6 cm. The diameter of the tumor was 11 mm (Figure S1). Although the surgeon experienced some difficulty in localizing the Magseed®, he successfully removed the Magseed® and the tumor with negative margins.
One disadvantage of this method is that, if deployed in the wrong place, the incorrectly placed seed would need to be surgically excised in addition to the correctly sited device.
For each patient, identification of the Magseed® markers in specimens was performed by the pathologist and by radiography whenever necessary. The pathologist reported some difficulties in finding the seed. A change in size or color would be desirable to optimize detection.
Other alternatives to WGL and Magseed® have become available. These include radio-guided occult lesion localization (ROLL) (23), RSL (24), the infrared radar technique SCOUT (20) and LOCalizer using radiofrequency identification (25). Each system has its advantages and disadvantages. For example, the main disadvantage of the ROLL and RSL techniques is the use of radioactivity. One particularity of the Magseed® marker, unlike the localization systems, is that it requires the use of plastic or titanium surgical instruments. The iron contained in metal instruments disrupts the Sentimag® probe. However, it is possible to use metal instruments just be sure to take away the instruments when the probe is in use so as not to interfere the signal. The large size of the SCOUT (12 mm) and the LOCalizer (11 mm) tags represents a disadvantage in comparison to the size of the Magseed® marker (5 mm) and RSL (4.5 mm) (26). In addition, these techniques are significantly more expensive than WGL leading to obstacles in broadening their use in practices. Davis et al. compare the cost device for wire localization and non-wire localization like I-125 radioactive seed, Radar reflector, Magnetic seed, radio frequency identification tag (26). A comparison of the price of each device clearly demonstrates the low cost of WGL. The average device cost for WGL is around 18 € which makes it an economical choice compared to other techniques except the carbon marking which has a similar cost. Regarding the ROLL technique a randomized study was performed an economic evaluation comparing ROLL with WGL (27). It appeared that ROLL is not more cost-effective than WGL (28). This study took into account all the medical costs associated with the technique as well as the changes in quality of life. Regarding the RSL technique, Zhang et al. demonstrated that RSL had lower costs than WGL for breast-conserving surgery. But the nuclear regulatory issues and management can increase the costs (29). Devices prices for the Magseed® system and the SCOUT technique are quite similar (around 300 €). The LOCalizer is a little more expensive. Moreover, it is important to take into account the price of the detector which also vary (15,000 €–25,000 €). Like all new techniques, these are always more expensive at the beginning. But the increase in the number of users will have a significant impact on the decrease in the price of devices. It would also seem that the price of devices is different from one country to another. It is also necessary to take into account the reimbursement of these devices by the insurance system of the country. Further studies should be performed to determine if these techniques would lead to more cost-effective care. The implementation of non-wire localization techniques allowed more efficient use of radiology resources. To evaluate these new techniques allowing localization of impalpable breast lesions, a National UK group collects all data from units in the United Kingdom through the iBRA-NET study (30-32). This will allow large scale evaluation of clinical outcomes, ensuring device safety and efficacy, to define new guidelines for pre-operative detection of subclinical breast lesions.
ConclusionsOther Section
In conclusion, this study reported our clinical experience on 100 consecutive patients with whom we used the Magseed® magnetic marker to localize non-palpable breast lesions. Compared to the wire-guided technique we have been using for many years, the Magseed® combined with the Sentimag® probe is a safe and effective method for identification of subclinical breast lesions and is effective when extended microcalcification clusters are present, or in targeting multiple tumors within the same breast. One of the major advantages of the Magseed® marker is that it can be placed in advance allowing disassociation of radiology and surgery schedules. Further research is required to validate efficacy of other long-term applications for patients who require neoadjuvant treatment.
AcknowledgmentsOther Section
We are grateful to Mrs. Jeghers and Mr. Sibret for their help in the realization of this study.
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-552/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-552/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-552/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-552/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the CHC MontLegia Ethics Committee (reference No. 20/40/1052) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all participants included.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Cady B, Stone MD, Schuler JG, et al. The new era in breast cancer. Invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg 1996;131:301-8. [Crossref] [PubMed]
- Nederend J, Duijm LEM, Louwman MWJ, et al. Impact of transition from analog screening mammography to digital screening mammography on screening outcome in The Netherlands: a population-based study. Ann Oncol 2012;23:3098-103. [Crossref] [PubMed]
- Altomare V, Guerriero G, Giacomelli L, et al. Management of nonpalpable breast lesions in a modern functional breast unit. Breast Cancer Res Treat 2005;93:85-9. [Crossref] [PubMed]
- Nagtegaal ID, Duffy SW. Reduction in rate of node metastases with breast screening: consistency of association with tumor size. Breast Cancer Res Treat 2013;137:653-63. [Crossref] [PubMed]
- Conant EF, Toledano AY, Periaswamy S, et al. Improving Accuracy and Efficiency with Concurrent Use of Artificial Intelligence for Digital Breast Tomosynthesis. Radiol Artif Intell 2019;1:e180096. [Crossref] [PubMed]
- Snider HC Jr, Morrison DG. Intraoperative ultrasound localization of nonpalpable breast lesions. Ann Surg Oncol 1999;6:308-14. [Crossref] [PubMed]
- Bilgen IG, Memis A, Ustun EE. The retrospective analysis of 550 nonpalpable breast lesions with localisation biopsy. Turk J Diagn Intervent Radiol 2002;8:487-95.
- Rahusen FD, Bremers AJ, Fabry HF, et al. Ultrasound-guided lumpectomy of nonpalpable breast cancer versus wire-guided resection: a randomized clinical trial. Ann Surg Oncol 2002;9:994-8. [Crossref] [PubMed]
- Cheang E, Ha R, Thornton CM, et al. Innovations in image-guided preoperative breast lesion localization. Br J Radiol 2018;91:20170740. [Crossref] [PubMed]
- Harvey JR, Lim Y, Murphy J, et al. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Cancer Res Treat 2018;169:531-6. [Crossref] [PubMed]
- Price ER, Khoury AL, Esserman LJ, et al. Initial clinical experience with an inductible magnetic seed system for preoperative breast lesion localization. AJR Am J Roentgenol 2018;210:913-7. [Crossref] [PubMed]
- Hersi AF, Eriksson S, Ramos J, et al. A combined, totally magnetic technique with a magnetic marker for non-palpable tumour localization superparamagnetic iron oxide nanoparticles for sentinel lymph node detection in breast cancer surgery. European Journal of Surgical Oncology 2019;45:544-9. [Crossref] [PubMed]
- Zacharioudakis K, Down S, Bholah Z, et al. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur J Surg Oncol 2019;45:2016-21. [Crossref] [PubMed]
- Crèvecoeur et al. Technique innovante de repérage et d’exérèse des lésions mammaires infra cliniques en utilisant un clip paramagnétique. 2019 Nov. poster C11. 41es Journées de la société française de sénologie et de pathologie mammaire (SFSPM). Available online: https://www.senologie.com/congres/Marseille-2019
- Thekkinkattil D, Kaushik M, Hoosein MM, et al. A prospective, single-arm, multicentre clinical evaluation of a new localisation technique using non-radioactive Magseeds for surgery of clinically occult breast lesions. Clin Radiol 2019;74:974.e7-974.e11. [Crossref] [PubMed]
- Powell M, Gate T, Kalake O, et al. Magnetic Seed Localization (Magseed) for excision of impalpable breast lesions-The North Wales experience. Breast J 2021;27:529-36. [Crossref] [PubMed]
- Pohlodek K, Sečanský P, Haluzová I, et al. Localization of impalpable breast lesions and detection of sentinel lymph nodes through magnetic methods. Eur J Radiol 2019;120:108699. [Crossref] [PubMed]
- McCamley C, Ruyssers N, To H, et al. Multicentre evaluation of magnetic technology for localisation of non-palpable breast lesions and targeted axillary nodes. ANZ J Surg 2021;91:2411-7. [Crossref] [PubMed]
- Chan BK, Wiseberg-Firtell JA, Jois RH, et al. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev 2015;2015:CD009206. [Crossref] [PubMed]
- Hayes MK. Update on Preoperative Breast Localization. Radiol Clin North Am 2017;55:591-603. [Crossref] [PubMed]
- Teichgraeber DC, Martaindale S, Omofoye TS, et al. Immediate Migration of Biopsy Clip Markers After Upright Digital Breast Tomosynthesis-Guided Vacuum-Assisted Core Biopsy. Acad Radiol 2020;27:204-9. [Crossref] [PubMed]
- Gera R, Tayeh S, Al-Reefy S, et al. Evolving Role of Magseed in Wireless Localization of Breast Lesions: Systematic Review and Pooled Analysis of 1,559 Procedures. Anticancer Res 2020;40:1809-15. [Crossref] [PubMed]
- Hawkins SC, Brown I, King P, et al. Time to go wireless? A 15-year single institution experience of radioisotope occult lesion localisation (ROLL) for impalpable breast lesions. Eur J Surg Oncol 2017;43:62-7. [Crossref] [PubMed]
- Milligan R, Pieri A, Critchley A, et al. Radioactive seed localization compared with wire-guided localization of non-palpable breast carcinoma in breast conservation surgery- the first experience in the United Kingdom. Br J Radiol 2018;91:20170268. [Crossref] [PubMed]
- Malter W, Holtschmidt J, Thangarajah F, et al. First Reported Use of the Faxitron LOCalizer™ Radiofrequency Identification (RFID) System in Europe - A Feasibility Trial, Surgical Guide and Review for Non-palpable Breast Lesions. In Vivo 2019;33:1559-64. [Crossref] [PubMed]
- Davis KM, Raybon CP, Monga N, et al. Image-guided localization techniques for non palpable breast lesions: an opportunity for multidisciplinary patient-centered care. Journal of Breast Imaging 2021;3:542-55. [Crossref]
- Postma EL, Verkooijen HM, van Esser S, et al. Efficacy of ‘radioguided occult lesion localisation’ (ROLL) versus ‘wire- guided localisation’ (WGL) in breast conserving surgery for non- palpable breast cancer: a randomised controlled multicentre trial. Breast Cancer Res Treat 2012;136:469-78. [Crossref] [PubMed]
- Postma EL, Koffijberg H, Verkooijen HM, et al. Cost-effectiveness of radioguided occult lesion localization (ROLL) versus wire-guided localization (WGL) in breast conserving surgery for nonpalpable breast cancer: results from a randomized controlled multicenter trial. Ann Surg Oncol 2013;20:2219-26. [Crossref] [PubMed]
- Zhang Y, Seely J, Cordeiro E, et al. Radioactive Seed Localization Versus Wire-Guided Localization for Nonpalpable Breast Cancer: A Cost and Operating Room Efficiency Analysis. Ann Surg Oncol 2017;24:3567-73. [Crossref] [PubMed]
- Somasundaram SK, Potter S, Elgammal S, et al. Impalpable breast lesion localisation, a logistical challenge: results of the UK iBRA-NET national practice questionnaire. Breast Cancer Res Treat 2021;185:13-20. [Crossref] [PubMed]
- Dave RV, Barrett E, Morgan J, et al. Wire- and magnetic-seed-guided localization of impalpable breast lesions: iBRA-NET localisation study. Br J Surg 2022;109:274-82. [Crossref] [PubMed]
- Morgan JL, Bromley HL, Dave RV, et al. Results of shared learning of a new magnetic seed localisation device - A UK iBRA-NET breast cancer localisation study. Eur J Surg Oncol 2022;48:2408-13. [Crossref] [PubMed]


