
The unifying concepts of the sick lobe hypothesis, field cancerisation and breast conservation treatment for multiple ipsilateral breast cancers: a narrative review
Introduction
For an extended period of time, the surgical treatment of breast cancer depended on the use of various forms of mastectomy, from the radical mastectomy, to modified radical and then total mastectomy. Although change was first suggested in the year 1969, it was not until some two decades later that breast conservation treatment (BCT) would be considered an appropriate alternative to mastectomy for the surgical treatment of breast cancer (1,2).
Initially, the proposal to de-escalate surgical treatment for breast malignancies in 1969 was met with a great deal of scepticism and caution by several prominent clinicians and pathologists of the time (3-5), who in turn described residual foci of carcinoma present after a simulated partial mastectomy, as well as the presence of multifocality and multicentricity in up to 63% of patients who were thought to have unifocal disease at presentation. With the publication of early results of the Milan I and NSABP B-06 trial in the year 1985 (6,7), one of these dissenters had to concede that it was possible that radiotherapy may eradicate or impair indefinitely the progress or clinical viability of these occult foci of disease (4), These early data supported surgical de-escalation and a consensus statement in the year 1991 established BCT as an appropriate alternative for women with breast cancer in the early operable stages (2). Twenty-year follow up of the NSABP B-06 study confirmed the ability of radiotherapy to effect local control similar for women who BCT as those who had total mastectomy (8). More recently, there is compelling evidence to show that BCT results in superior survival (9-33). There are also reports of survival comparison between BCT and mastectomy in certain specific circumstances (34-45). These include studies analysing patients treated with neoadjuvant chemotherapy, women younger than 40 years and those with node negative disease. Consistently, even in these distinct clinical situations, survival in women undergoing BCT is reported to be superior to those who had undergone mastectomy. As such, there are several reviews and commentaries supporting the findings of these studies (46-51).
This purpose of this review is to synthesise historical with contemporary data on BCT for multiple ipsilateral breast cancer (MIBC), also referred to as multifocal multicentric breast cancer (MFMCBC), and to apply the information as a basis for future research. We present this article in accordance with the Narrative Review reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-609/rc).
Methods
A PubMed search was performed for articles published from 1970 to November 2022 in the English language with the terms: ‘multifocal multicentric breast cancer AND breast conservation treatment’ (101 articles), ‘multiple ipsilateral breast cancer AND breast conservation treatment’ (66 articles), ‘sick lobe hypothesis’ (15 articles), and ‘field cancerisation AND genetic changes AND breast margins’ (10 articles) (Table 1, Figure 1). Of the articles searched, exclusion criteria comprised articles on male breast cancer, non-carcinomatous lesions like phyllodes tumours, radiotherapy, axillary dissection, focussed discussion on breast imaging or oncoplastic surgery independent of MIBC. The search was then reviewed for duplication. Specific commentaries, supporting articles, cohort studies, prospective studies with analyses on MFMCBC or MIBC and recent articles with guidelines on the surgical treatment of breast cancer were additionally included in this review.
Table 1
| Items | Specifications |
|---|---|
| Date of search | 6 December 2022 |
| Database search | PubMed |
| Search terms | Multifocal multicentric breast cancer AND breast conservation treatment (101 citations) |
| Multiple Ipsilateral breast cancer AND breast conservation treatment (66 citations) | |
| Sick lobe hypothesis (15 citations) | |
| Field cancerisation AND genetic changes AND breast margins (10 citations) | |
| Time frame | 1970 to present |
| Language | English language |
| Inclusion criteria | Relevant to subject discussed |
| Exclusion criteria | Male breast cancer, non-carcinomatous lesions like phyllodes tumours, radiotherapy, axillary dissection, focussed discussion on imaging, oncoplastic surgery independent of MIBC and duplications |
| Selection process | Both authors reviewed citations and selected relevant articles based on consensus agreement |
MIBC, multiple ipsilateral breast cancer.
Discussion
Preamble
Prior to the published results of the early prospective randomised controlled trials (RCTs) comparing BCT with mastectomy, there was already pathologic information regarding the existence of multifocality and multicentricity of breast malignancy (3-8). Of interest, these RCTs were conducted in the era prior to the routine use of sophisticated imaging techniques like magnetic resonance imaging (MRI) to detect occult tumour foci. Yet, it was found that there was no difference in overall survival and in the NSABP B-06 study, data suggested that local recurrence rates were numerically lower for women who underwent lumpectomy with radiotherapy than for those who underwent mastectomy (8). These results of similar survival and local control presented the prospect of the use of BCT as a reasonable approach for the treatment of MFMCBC.
Definition and nomenclature of multiple ipsilateral breast carcinoma
Various non-standardised definitions of multifocal (MF) and multicentric (MC) breast lesions have been reported (52). Some have referred to MF disease as two or more distinct lesions occurring in the same quadrant, while MC disease refers to the presence of multiple tumour foci in more than one quadrant (52,53). Distance between lesions (ranging from 2 to 5 cm) were also used to define multiplicity of tumour foci less frequently in the past (53). However, more recently, situations where there are more than one foci identified in the ipsilateral breast are collectively referred to as MIBC and in a recent trial, an intervening normal appearing tissue of 2 cm between two malignant foci was used as the inclusion criteria (54).
Evidence for BCT for MIBC
Early studies with BCT for MIBC reported unacceptably high local recurrence rates. However, with time, more recent articles have shown recurrence rates similar to those for unifocal disease (55-59). In one of the earlier studies, Cho et al. indicated that it was the exceptional patient who would qualify for BCT with macroscopically multiple ipsilateral invasive breast cancers (60). However, with time and the accumulation of further data, a consensus by an expert panel endorsed the use of BCT for MIBC provided clear margins were obtained for each foci, whole breast radiotherapy was planned and a reasonable cosmetic outcome was achieved (61,62). The presence of multiple ipsilateral foci of mammary malignancy is a poor prognostic indicator; however, higher locoregional and distant relapse was observed independently from the type of surgery performed (63-65). Hence, there are now several authors who support the use of BCT as a safe surgical treatment option for MIBC (66,67).
The sick lobe hypothesis and MIBC
Early cadaveric studies by Sir Astley Cooper demonstrated the lobar distribution of the ductal system (68,69). The use of mastectomy for the treatment of breast cancer overshadowed and suppressed the application of this knowledge in surgery. However, with contemporary data demonstrating superior outcomes with BCT (9) and surgical diminution coming to the fore, it is now appropriate to review how the lobar arrangement in mammary anatomy is relevant in the treatment of breast cancer.
There was a failure to recognise how Cooper’s seminal work on breast anatomy was intimately related to the distribution of multiplicity of tumour foci when multifocality and multicentricity of breast cancer was first reported (3-5). However, there is now sufficient evidence to demonstrate its importance in the understanding of MFMCBC and how adopting an approach which combines anatomy, modern pathology and molecular information can determine the optimum surgical approach for local control.
The terminal duct lobular units are structured such that they are arranged radially in 15–20 lobes, from a central convergence in the nipple. This can be traced to embryological origins of the breast (70-73). Two rows of cuboidal epithelial cells, with fibroblasts, mesothelial cells and a basal cell layer surround lumina which arborise from the central lactiferous ampullae, eventually converge to form the nipple. This duct anatomy is relevant to the neoplastic process (74), for it has been seen that both ductal and lobular carcinoma (DCIS/LCIS), have disease patterns which align with the sick lobe concept (75). Large section, or subgross histology studies have shown that a significant proportion of malignant lesions demonstrate similar MF, MC and diffuse arrangements (76,77). The sick lobe hypothesis proposed by Going and Tot adduces that breast carcinoma is fundamentally a lobar disease (75,78). Genetic instability through mutation is thought to be initiated during embryonic development, with precursor cells transmitting this characteristic to their subsequent generations within the distribution of the entire lobe with further developmental progress (79). During maturation, deleterious cellular or stromal events may occur and contribute to further mutations, and the additive result of these factors lead eventually to malignant transformation. Due to the distribution of cells with vulnerable genetic alterations within the same ductal tree, multiple tumour foci may originate, simultaneously or asynchronously, from epithelial cells within a single lobe of the breast. These may originate within the main duct or the terminal ductal-lobular units (80,81). This forms what is referred to as MF disease. Synchronous malignant transformation occurring in two or more sick lobes are denoted as MC disease.
The ‘at risk’ population of cells have been shown to occupy a conical configuration with the apex directed to the nipple-areola complex, which is consistent with the estimated architectural arrangement of a single ‘sick lobe’ (77,78). The radial lobes forming a pyramidal shape within the breast comprise of individual duct systems which may vary significantly in size, overlie one another and present with variations of segmental, peripheral or diffuse patterns. This theory is supported by what is currently known in the sphere of molecular evolution of breast cancers, where similar genetic changes are demonstrated in both progenitor lesions and subsequent malignant tumours occupying the estimated distribution of the affected lobe(s) (80,81). An analysis of genetic alterations in homogenous phenotypic ductal MF lesions on the basis of various characteristics including oestrogen receptor (ER), human epidermal growth factor receptor 2 (HER2) status and grade demonstrated three ‘genomic’ groups: a ‘homogeneous’ group where all MF lesions carried the same mutations, an ‘intermediate group’, with both common and private mutations, and a ‘heterogeneous’ group without common mutations (82). The single significant factor between inter-lesional heterogeneity and clinico-pathological characteristics was inter-lesional distance. Patients within the homogenous group had lesions closer to each other than those in the heterogeneous group. Once again, this observation is consistent with the concept of the sick lobe(s), where a greater degree of homogeneity of molecular alterations in lesions in closer proximity is indicative of common embryologic ancestry, while genetic heterogeneity in more distant tumour foci suggests that they are derived from different ductal-lobular trees with separate genetic origins. It would be reasonable to infer from these findings that anatomic architecture and genetic distribution of affected lobes should be a critical consideration when performing surgical resection.
Field cancerisation and surgical resection volumes
There is contemporary evidence supporting a succedent, multi-step genetic model of oncogenesis beginning with a single cell first acquiring one or more genetic or epigenetic aberrations, allowing it a proliferative advantage, leading to the formation of a clonal field of similarly altered cells (83). In its earliest form, histologic architecture may not be disrupted. The precursor field enlarges as proliferation generates more altered cells with some but not all genetic changes acquired for frank malignant transformation. This constitutes a ‘cancerised field’ with a propensity for further progression to malignancy and correlates well with the sick lobe hypothesis. It has been observed that phenotypically- normal appearing epithelial tissue that bear ‘hallmarks of cancer’ are detected within a 1 cm radius from breast tumours, but not in tissues 5 cm from tumour (83), implying different lobar origins as distance from tumour foci increase. Detection of such molecular changes, or their absence, therefore, could be used as markers for adequacy of tissue resection.
Apart from the distribution of altered epithelial cells within the sick lobe, its surrounding stroma and associated microenvironment may have implications for surgical resection volume. Epithelial to mesenchymal transition in epithelial cells, telomerase expression, genomic instability and myofibroblasts, associated with dense disorganised extracellular matrix are thought to be drivers of tumour initiation and progression (83). In addition, they may contribute to tumour recurrence. Therefore, excision of such phenotypically normal but genetically ‘primed’ tissue can have a positive impact on local control and possibly reduce recurrence. In combination, the extent of the diseased ductal-lobular tree (sick lobe) and adjacent affected stroma, considered the involved ‘segment’, may be used to determine markers and volume estimations for adequate resection. Using tumour morphology and characteristics as histological surrogates for mechanistic parameters, a mathematical model may be derived to predict tumour volumes (44). Such calculations made preoperatively can enhance the accuracy of surgical resection volume. There may be variations in the geometry and distribution of intraductal tumour cells in different patients but using a composite of imaging and pathologic characteristics, a formula may be used to calculate estimated disease extent for individualised surgical planning. This formula, as proposed by Edgerton et al. (84), predicts resection volume in the shape of an ellipse, which is consistent with the expected distribution of the sick lobe to a large extent.
Excising the ‘sick segment’ poses the dilemma of what constitutes a negative margin. The current consensus for a clear margin is ‘no ink on tumour’ for invasive disease and some experts advocate 2 mm margins for ductal carcinoma in situ (DCIS) (61,62). The concept of the sick segment with identification of molecular changes at the margins of histologically normal tissue introduces another dimension which may appear contradictory. A change in definition of a clear margin is not propounded at this point in time. However, the authors suggest that more research is required in this area, and there may be a role for de-escalating radiotherapy if marginal tissue do not demonstrate molecular changes which confer predilection for carcinogenesis. This enables individualised treatment, just as genomic signatures inform systemic therapy.
Implications of the sick lobe hypothesis and field cancerisation for BCT in MIBC
With contemporary evidence that BCT confers superior survival outcomes (9), the objective of modern surgical treatment of breast cancer should be minimising the need for mastectomy. A significant body of literature exists on how oncoplastic breast surgery (OBS) can expand the indications for BCT while offering reasonable cosmesis (85). OBS is a collective term for a myriad of operative techniques but many of these techniques are complex and complicated and may not achieve optimum ‘de-escalation’ of surgery. OBS has been shown to be associated with increased complications with no significant benefit in local control (86). Moreover, the resection techniques employed in OBS prescribe either a circular or spherical resection volume around the tumour (87), which is not consistent with the anatomy of the sick lobe and the elliptical resection proposed by Edgerton et al. The spherical or cylindrical surgical techniques not only fail to respect the patterns of tumour propagation and distribution but the defect created in this fashion actually hinders direct parenchymal closure, requiring more complicated procedures to fill the defect created by tumour resection (87).
Perhaps a more appropriate approach would be to apply standard breast conservation surgery (sBCS) utilising the principles of resecting the sick lobe and cancerised field. Following the strategy proposed by Edgerton, an elliptical resection optimises removal of the ‘sick lobe’ and the shape of the resection defect can be closed by the less complex manoeuvre of full thickness parenchymal flap mobilisation, followed by direct closure (88,89). This de-escalation of OBS to sBCS appropriately excises the ‘sick segment’ (lobe with anticipated cancerised field) with adequate margins, reduces surgical complications and optimises cosmetic outcomes. Lobar surgery adheres to these principles and may offer an optimum surgical approach for resection of MIBC (90).
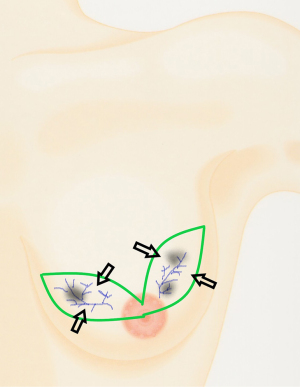
In the presence of MC tumours, an excision with margins of two elliptical sections of breast tissue connected by a retroareolar bridge of tissue may effectively remove the involved sick lobes and cancerised fields (Figure 2). Such an approach is termed a ‘multisegment tissue resection pattern’, and the resulting defect may be restored though standard parenchymal closure using tissue displacement techniques only (89) (Figure 2). This is the fundamental principle behind lobar surgery for MIBC and does not require complex and complicated tissue replacement or therapeutic mammoplasty techniques (90). Approximately 85% of patients with MIBC can undergo sBCS without oncoplastic procedures and have a satisfactory or better cosmetic outcome (91). The minority of patients who require resection in excess of 20% of total breast tissue volume may require therapeutic mammoplasty, volume replacement procedures or mastectomy with or without reconstruction.
Optimising BCT rates for MIBC
Increased identification of MIBC was possible through modern imaging. Conventional imaging with mammogram and sonography detect multiple tumours in approximately 20–25% of patients with breast cancer (92). MRI should be used with caution and only in selected patients, as its routine use carries the risk of a higher likelihood of a patient undergoing mastectomy for no demonstrable outcome advantages in terms of fewer re-excisions or improved local control as adjuvant whole breast radiotherapy effectively controls occult tumour foci undetected by conventional imaging (93). A policy for restricted and sparing use of MRI should therefore translate to higher BCT rates for MIBC and reduces over-surgery for breast cancer (94). The authors apply MRI only if there is strong clinical suspicion that conventional imaging with mammogram and ultrasound has not adequately delineated the extent of disease.
Neoadjuvant treatment is able to effect tumour downstaging to convert a patient who is assessed to be ineligible for BCT at initial presentation to eligibility after a course of preoperative chemotherapy (95). There is no impairment of disease-free and overall survival for such patients who undergo BCT after neoadjuvant chemotherapy (96). The conversion rate from mastectomy to BCT has been reported to be 75% of patients with T1-3 tumours, and a similar de-escalation of patients with MIBC initially assessed to require mastectomy is expected, although there is insufficient confirmatory data at this point in time. However, it is worth mentioning that all clinically and imaging evident tumour foci, as well as potentially involved axillary lymph nodes should be marked with a clip or other device for tumour site localisation at surgery following neoadjuvant therapy.
Future directions for BCT in MIBC
Classical anatomy and histology studies have provided the fundamental basis for the understanding of the distribution of MIBC. The more contemporary science of genetics offers further insights into how molecular alterations affects tumour development, progress and clinical implications (97). Apart from molecular modifications in the epithelial cells, stromal changes in the vicinity of the ductal-lobular tree may instigate tumour progression, recurrence and offer prognostic information. These characteristics may be used in combination for local as well as systemic treatment planning.
In addition to these factors, the presence of tumour infiltrating lymphocytes have been shown to be associated with higher rates of complete pathologic response with neoadjuvant chemotherapy, and this may be a contributing factor to better survival outcomes (98). Immune response having a synergistic effect may have implications not only for primary medical therapy, but for surgery as well. More extensive operative procedures have been suggested to confer a more profound negative impact on the individual’s immune system and hence poorer survival outcomes for patients with mastectomy (48). Logically, then, the less extensive surgical modality of sBCS would result in a lower level of immune disruption and could potentially explain the improved survival seen with BCT. However, ethical considerations may make such an analysis in the form of a prospective RCT improbable. Nevertheless, there is sufficient data at present for future study into the carcinogenic effect of various genetic aberrations occurring within the ‘sick segment’, and the impact of its adequate resection on local control and systemic therapy. This can further enhance what has already been achieved with augmented reality volume estimation of tumour resection (99).
Medicine has moved into the era of immunotherapy and functional imaging, each of which has been used for more than a decade. Combining the concepts from these available technologies, we envisage that it would be possible to develop a physiological substrate which might tag the genetic change(s) identified on preoperative core biopsy. Using this, a functional imaging modality similar to positron emission tomography (PET scan) might be used to locate the sites at which the substrate has attached to within the breast. Using augmented reality, just as Laas et al. have described, an estimated resection volume can be superimposed onto an avatar of the patient and used as a guide for surgery (99). Although much research is required before this can become a reality, the authors believe that this development is possible in the not too distant future.
There are ongoing clinical studies on BCT for MIBC. The data for the American College of Surgical Oncology Group Z 11102 (Alliance) study, which is a single arm cohort study evaluating local recurrence for women with MIBC undergoing BCT is expected to mature in the year 2022 (54). The MIAMI (multiple ipsilateral breast conserving surgery versus mastectomy) trial in the United Kingdom is a prospective feasibility multicentre trial for the comparison of BCT versus mastectomy. However, the investigators for the MIAMI trial have reported dismal accrual to date as women eligible for the trial are resistant to randomisation (100). Some patients actively decline mastectomy. This represents a significant shift in attitudes. When the concept of BCT for MIBC was first mooted a few decades ago, clinicians reported that it would be the exceptional patient who would qualify for conservative surgery (60). However, women with MIBC now are more inclined to undergo BCT (100). The investigators for MIAMI anticipate that it would be extremely challenging to proceed with a full scale prospective RCT and suggest funding for prospective cohort studies instead (100).
In the light of these challenges, the concept of the sick lobe hypothesis and field cancerisation in combination, offers insights which have yet to be fully researched and recognised; for it may prove to be invaluable as a surrogate for a trial comparing BCT with mastectomy, where its potential can be exploited for individualised medical treatment, radiotherapy and selection criteria for precision surgery in the treatment of breast cancer (Table 2).
Table 2
| Concept | References | Year published | Relevance & comments |
|---|---|---|---|
| Anatomy of the breast with radial arrangement of ducto-lobular segments | (68,69) | 1840 | Offers insights into distribution of breast cancer |
| Multifocality & multicentricity of breast malignancy demonstrated within mastectomy specimens, performed for what was thought to be unifocal tumours clinically | (3-5) | 1975–1985 | Caution expressed when surgeons were embarking on prospective trials |
| Randomised controlled trials showing similar survival and reasonable local control results with BCT for clinically unifocal disease without the use of MRI | (2,5-8) | 1985–2002 | Radiotherapy possibly eradicated subclinical foci of tumours undetected by conventional imaging of the day |
| Early studies showed unacceptable recurrence rates | (55,56,58) | 1989–1993 | Adequacy and appropriate treatment needs to be applied |
| More recent studies with larger patient cohorts demonstrating reasonable local control | (12,19,57-60) | 1997–2009 | Better understanding of requirements for clear margins, surgical techniques for good cosmetic outcomes and whole breast radiotherapy likely contributing factors to good results for local control |
| (13-18,21,22,29,63,64,92) | 2011–2015 | ||
| (20,23-28,30-36,46-50,65) | 2016–2019 | ||
| (37-45,51) | 2020–2022 | ||
| Sick lobe hypothesis discussed | (74-78) | 1996–2016 | Large section histopathology provided insights into the tumour distribution within the sick lobe |
| Molecular changes within tumour tissue and concept of field cancerisation | (81-84) | 2010–2017 | Adjacent tissue which may be histologically normal may have genetic changes predisposing epithelial cells to undergo carcinogenesis |
| Guidelines endorsing the use of BCT for MFMCBC/MIBC | (61,62) | 2015–2017 | No longer discussed at later St Gallen International Breast Cancer Conferences (from the year 2019 onwards), indicating acceptance of BCT for MFMCBC/MIBC when listed criteria met |
| Commentaries approving the use of BCT for MFMCBC/MIBC | (66,67) | 2019,2020 | Analysis of contemporary data shows no detrimental impact when BCT applied for the treatment of MFMCBC/MIBC |
| Synthesis of the sick lobe hypothesis and field cancerisation concepts for optimal resection in MFMCBC/MIBC | Current article | 2022 | Paves the way for de-escalation of surgery, reducing over-use of mastectomy and complex oncoplastic breast surgery in BCT for MFMCBC/MIBC |
MIBC, multiple ipsilateral breast cancer; BCT, breast conservation treatment; MRI, magnetic resonance imaging; MFMCBC, multifocal multicentric breast cancer.
Conclusions
The sick segment concept, which is a combination of the sick lobe hypothesis and field cancerisation, offers rationale for local treatment of breast cancer and future directions for research into optimising therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-609/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-609/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-609/coif). MPT serves as an unpaid editorial board member of Gland Surgery from June 2022 to May 2024. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- NIH consensus conference. Treatment of early-stage breast cancer. JAMA 1991;265:391-5. [Crossref] [PubMed]
- Rosen PP, Fracchia AA, Urban JA, et al. "Residual" mammary carcinoma following simulated partial mastectomy. Cancer 1975;35:739-47. [Crossref] [PubMed]
- Lagios MD. Multicentricity of breast carcinoma demonstrated by routine correlated serial subgross and radiographic examination. Cancer 1977;40:1726-34. [Crossref] [PubMed]
- Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer 1985;56:979-90. [Crossref] [PubMed]
- Veronesi U, Zucali R, Del Vecchio M. Conservative treatment of breast cancer with QU.A.R.T. Technique. World J Surg 1985;9:676-81. [Crossref] [PubMed]
- Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665-73. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- De la Cruz Ku G, Karamchandani M, Chambergo-Michilot D, et al. Does Breast-Conserving Surgery with Radiotherapy have a Better Survival than Mastectomy? A Meta-Analysis of More than 1,500,000 Patients. Ann Surg Oncol 2022;29:6163-88. [Crossref] [PubMed]
- van Hezewijk M, Bastiaannet E, Putter H, et al. Effect of local therapy on locoregional recurrence in postmenopausal women with breast cancer in the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial. Radiother Oncol 2013;108:190-6. [Crossref] [PubMed]
- van der Heiden-van der Loo M, Siesling S, Wouters MW, et al. The Value of Ipsilateral Breast Tumor Recurrence as a Quality Indicator: Hospital Variation in the Netherlands. Ann Surg Oncol 2015;22:S522-8. [Crossref] [PubMed]
- Martin MA, Meyricke R, O'Neill T, et al. Breast-conserving surgery versus mastectomy for survival from breast cancer: the Western Australian experience. Ann Surg Oncol 2007;14:157-64. [Crossref] [PubMed]
- Bantema-Joppe EJ, de Munck L, Visser O, et al. Early-stage young breast cancer patients: impact of local treatment on survival. Int J Radiat Oncol Biol Phys 2011;81:e553-9. [Crossref] [PubMed]
- Hwang ES, Lichtensztajn DY, Gomez SL, et al. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 2013;119:1402-11. [Crossref] [PubMed]
- Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014;149:267-74. [Crossref] [PubMed]
- Hofvind S, Holen Å, Aas T, et al. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol 2015;41:1417-22. [Crossref] [PubMed]
- Hartmann-Johnsen OJ, Kåresen R, Schlichting E, et al. Survival is Better After Breast Conserving Therapy than Mastectomy for Early Stage Breast Cancer: A Registry-Based Follow-up Study of Norwegian Women Primary Operated Between 1998 and 2008. Ann Surg Oncol 2015;22:3836-45. [Crossref] [PubMed]
- Saadatmand S, Bretveld R, Siesling S, et al. Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173,797 patients. BMJ 2015;351:h4901. [Crossref] [PubMed]
- Morris AD, Morris RD, Wilson JF, et al. Breast-conserving therapy vs mastectomy in early-stage breast cancer: a meta-analysis of 10-year survival. Cancer J Sci Am 1997;3:6-12. [PubMed]
- van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158-70. [Crossref] [PubMed]
- Keating NL, Landrum MB, Brooks JM, et al. Outcomes following local therapy for early-stage breast cancer in non-trial populations. Breast Cancer Res Treat 2011;125:803-13. [Crossref] [PubMed]
- Brooks JM, Chrischilles EA, Landrum MB, et al. Survival implications associated with variation in mastectomy rates for early-staged breast cancer. Int J Surg Oncol 2012;2012:127854. [Crossref] [PubMed]
- Plichta JK, Rai U, Tang R, et al. Factors Associated with Recurrence Rates and Long-Term Survival in Women Diagnosed with Breast Cancer Ages 40 and Younger. Ann Surg Oncol 2016;23:3212-20. [Crossref] [PubMed]
- Mogal HD, Clark C, Dodson R, et al. Outcomes After Mastectomy and Lumpectomy in Elderly Patients with Early-Stage Breast Cancer. Ann Surg Oncol 2017;24:100-7. [Crossref] [PubMed]
- Nandakumar A, Rath GK, Kataki AC, et al. Decreased Survival With Mastectomy Vis-à-Vis Breast-Conserving Surgery in Stage II and III Breast Cancers: A Comparative Treatment Effectiveness Study. J Glob Oncol 2017;3:304-13. [Crossref] [PubMed]
- Hartmann-Johnsen OJ, Kåresen R, Schlichting E, et al. Better survival after breast-conserving therapy compared to mastectomy when axillary node status is positive in early-stage breast cancer: a registry-based follow-up study of 6387 Norwegian women participating in screening, primarily operated between 1998 and 2009. World J Surg Oncol 2017;15:118. [Crossref] [PubMed]
- Christiansen P, Carstensen SL, Ejlertsen B, et al. Breast conserving surgery versus mastectomy: overall and relative survival-a population based study by the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol 2018;57:19-25. [Crossref] [PubMed]
- Lagendijk M, van Maaren MC, Saadatmand S, et al. Breast conserving therapy and mastectomy revisited: Breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer 2018;142:165-75. [Crossref] [PubMed]
- Fisher S, Gao H, Yasui Y, et al. Survival in stage I-III breast cancer patients by surgical treatment in a publicly funded health care system. Ann Oncol 2015;26:1161-9. [Crossref] [PubMed]
- Kim K, Park HJ, Shin KH, et al. Breast Conservation Therapy Versus Mastectomy in Patients with T1-2N1 Triple-Negative Breast Cancer: Pooled Analysis of KROG 14-18 and 14-23. Cancer Res Treat 2018;50:1316-23. [Crossref] [PubMed]
- Fakhreddine MH, Haque W, Ahmed A, et al. Prognostic Factors, Treatment, and Outcomes in Early Stage, Invasive Papillary Breast Cancer: A SEER Investigation of Less Aggressive Treatment in a Favorable Histology. Am J Clin Oncol 2018;41:532-7. [Crossref] [PubMed]
- de Boniface J, Frisell J, Bergkvist L, et al. Breast-conserving surgery followed by whole-breast irradiation offers survival benefits over mastectomy without irradiation. Br J Surg 2018;105:1607-14. [Crossref] [PubMed]
- Wang J, Wang S, Tang Y, et al. Comparison of Treatment Outcomes With Breast-conserving Surgery Plus Radiotherapy Versus Mastectomy for Patients With Stage I Breast Cancer: A Propensity Score-matched Analysis. Clin Breast Cancer 2018;18:e975-84. [Crossref] [PubMed]
- Arlow RL, Paddock LE, Niu X, et al. Breast-conservation Therapy After Neoadjuvant Chemotherapy Does Not Compromise 10-Year Breast Cancer-specific Mortality. Am J Clin Oncol 2018;41:1246-51. [Crossref] [PubMed]
- Corradini S, Reitz D, Pazos M, et al. Mastectomy or Breast-Conserving Therapy for Early Breast Cancer in Real-Life Clinical Practice: Outcome Comparison of 7565 Cases. Cancers (Basel) 2019;11:160. [Crossref] [PubMed]
- Lazow SP, Riba L, Alapati A, et al. Comparison of breast-conserving therapy vs mastectomy in women under age 40: National trends and potential survival implications. Breast J 2019;25:578-84. [Crossref] [PubMed]
- Almahariq MF, Quinn TJ, Siddiqui Z, et al. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother Oncol 2020;142:186-94. [Crossref] [PubMed]
- Wrubel E, Natwick R, Wright GP. Breast-Conserving Therapy is Associated with Improved Survival Compared with Mastectomy for Early-Stage Breast Cancer: A Propensity Score Matched Comparison Using the National Cancer Database. Ann Surg Oncol 2021;28:914-9. [Crossref] [PubMed]
- Wen S, Manuel L, Doolan M, et al. Effect of Clinical and Treatment Factors on Survival Outcomes of Triple Negative Breast Cancer Patients. Breast Cancer (Dove Med Press) 2020;12:27-35. [Crossref] [PubMed]
- Akbari ME, Khayamzadeh M, Mirzaei HR, et al. Saving the Breast Saves the Lives of Breast Cancer Patients. Int J Surg Oncol 2020;2020:8709231. [Crossref] [PubMed]
- Chu QD, Hsieh MC, Lyons JM, et al. 10-Year Survival after Breast-Conserving Surgery Compared with Mastectomy in Louisiana Women with Early-Stage Breast Cancer: A Population-Based Study. J Am Coll Surg 2021;232:607-21. [Crossref] [PubMed]
- Wan Q, Su L, Ouyang T, et al. Comparison of Survival After Breast-Conserving Therapy vs Mastectomy Among Patients With or Without the BRCA1/2 Variant in a Large Series of Unselected Chinese Patients With Breast Cancer. JAMA Netw Open 2021;4:e216259. [Crossref] [PubMed]
- Chu QD, Hsieh MC, Yi Y, et al. Outcomes of Breast-Conserving Surgery Plus Radiation vs Mastectomy for All Subtypes of Early-Stage Breast Cancer: Analysis of More Than 200,000 Women. J Am Coll Surg 2022;234:450-64. [Crossref] [PubMed]
- Saifi O, Chahrour MA, Li Z, et al. Is breast conservation superior to mastectomy in early stage triple negative breast cancer? Breast 2022;62:144-51. [Crossref] [PubMed]
- Li P, Li L, Xiu B, et al. The Prognoses of Young Women With Breast Cancer (≤35 years) With Different Surgical Options: A Propensity Score Matching Retrospective Cohort Study. Front Oncol 2022;12:795023. [Crossref] [PubMed]
- Johns N, Dixon JM. Should patients with early breast cancer still be offered the choice of breast conserving surgery or mastectomy? Eur J Surg Oncol 2016;42:1636-41. [Crossref] [PubMed]
- McCormick B. The mastectomy myth. Lancet Oncol 2016;17:1035-7. [Crossref] [PubMed]
- Gentilini OD, Cardoso MJ, Poortmans P. Less is more. Breast conservation might be even better than mastectomy in early breast cancer patients. Breast 2017;35:32-3. [Crossref] [PubMed]
- Fancellu A. The need to diminish mastectomy rates in patients with breast cancer eligible for breast conservation. Updates Surg 2019;71:597-8. [Crossref] [PubMed]
- Hartmann-Johnsen OJ. Breast-conserving therapy is better than mastectomy: based on registry data from Norway. University of Oslo; 2019.
- Murphy J, Gandhi A. Does Mastectomy Reduce Overall Survival in Early Stage Breast Cancer? Clin Oncol (R Coll Radiol) 2021;33:440-7. [Crossref] [PubMed]
- Kapoor NS, Chung A, Huynh K, et al. Preliminary results: double lumpectomies for multicentric breast carcinoma. Am Surg 2012;78:1345-8. [Crossref] [PubMed]
- Patani N, Carpenter R. Oncological and aesthetic considerations of conservational surgery for multifocal/multicentric breast cancer. Breast J 2010;16:222-32. [Crossref] [PubMed]
- Rosenkranz KM, Ballman K, McCall L, et al. The Feasibility of Breast-Conserving Surgery for Multiple Ipsilateral Breast Cancer: An Initial Report from ACOSOG Z11102 (Alliance) Trial. Ann Surg Oncol 2018;25:2858-66. [Crossref] [PubMed]
- Kurtz JM, Jacquemier J, Amalric R, et al. Breast-conserving therapy for macroscopically multiple cancers. Ann Surg 1990;212:38-44. [Crossref] [PubMed]
- Wilson LD, Beinfield M, McKhann CF, et al. Conservative surgery and radiation in the treatment of synchronous ipsilateral breast cancers. Cancer 1993;72:137-42. [Crossref] [PubMed]
- Kaplan J, Giron G, Tartter PI, et al. Breast conservation in patients with multiple ipsilateral synchronous cancers. J Am Coll Surg 2003;197:726-9. [Crossref] [PubMed]
- Carpenter S, Fraser J, Fleming M, et al. Optimal treatment of multiple ipsilateral primary breast cancers. Am J Surg 2008;196:530-6. [Crossref] [PubMed]
- Gentilini O, Botteri E, Rotmensz N, et al. Conservative surgery in patients with multifocal/multicentric breast cancer. Breast Cancer Res Treat 2009;113:577-83. [Crossref] [PubMed]
- Cho LC, Senzer N, Peters GN. Conservative surgery and radiation therapy for macroscopically multiple ipsilateral invasive breast cancers. Am J Surg 2002;183:650-4. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2017;28:1700-12. [Crossref] [PubMed]
- Neri A, Marrelli D, Megha T, et al. Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: a retrospective study on a series of 1158 cases BMC Surg 2015;15:1. [Crossref] [PubMed]
- Shaikh T, Tam TY, Li T, et al. Multifocal and multicentric breast cancer is associated with increased local recurrence regardless of surgery type. Breast J 2015;21:121-6. [Crossref] [PubMed]
- Fang M, Zhang X, Zhang H, et al. Local Control of Breast Conservation Therapy versus Mastectomy in Multifocal or Multicentric Breast Cancer: A Systematic Review and Meta-Analysis. Breast Care (Basel) 2019;14:188-93. [Crossref] [PubMed]
- Benson JR, Jatoi I, Toi M. Surgical management of multiple ipsilateral breast cancers. Future Oncol 2019;15:1185-91. [Crossref] [PubMed]
- Masannat YA, Agrawal A, Maraqa L, et al. Multifocal and multicentric breast cancer, is it time to think again? Ann R Coll Surg Engl 2020;102:62-6. [Crossref] [PubMed]
- Cooper AP. The anatomy of the breast. London: Longman; 1840.
- Cooper AP. Plates of the anatomy of the breast. London: Longman; 1840.
- Gusterson BA, Stein T. Human breast development. Semin Cell Dev Biol 2012;23:567-73. [Crossref] [PubMed]
- Lemaine V, Simmons PS. The adolescent female: Breast and reproductive embryology and anatomy. Clin Anat 2013;26:22-8. [Crossref] [PubMed]
- Richert MM, Schwertfeger KL, Ryder JW, et al. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 2000;5:227-41. [Crossref] [PubMed]
- Musumeci G, Castrogiovanni P, Szychlinska MA, et al. Mammary gland: From embryogenesis to adult life. Acta Histochem 2015;117:379-85. [Crossref] [PubMed]
- Moffat DF, Going JJ. Three dimensional anatomy of complete duct systems in human breast: pathological and developmental implications. J Clin Pathol 1996;49:48-52. [Crossref] [PubMed]
- Tot T. DCIS, cytokeratins, and the theory of the sick lobe. Virchows Arch 2005;447:1-8. [Crossref] [PubMed]
- Tot T, Gere M. Radiologically unifocal invasive breast carcinomas: large-section histopathology correlate and impact on surgical management. J Cancer Sci Ther 2016;8:50-4. [Crossref]
- Tot T. The role of large-format histopathology in assessing subgross morphological prognostic parameters: a single institution report of 1000 consecutive breast cancer cases. Int J Breast Cancer 2012;2012:395415. [Crossref] [PubMed]
- Going JJ, Mohun TJ. Human breast duct anatomy, the 'sick lobe' hypothesis and intraductal approaches to breast cancer. Breast Cancer Res Treat 2006;97:285-91. [Crossref] [PubMed]
- Agelopoulos K, Buerger H, Brandt B. Allelic imbalances of the egfr gene as key events in breast cancer progression--the concept of committed progenitor cells. Curr Cancer Drug Targets 2008;8:431-45. [Crossref] [PubMed]
- Tot T. Early and more advanced unifocal and multifocal breast carcinomas and their molecular phenotypes. Clin Breast Cancer 2011;11:258-63. [Crossref] [PubMed]
- Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, et al. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology 2010;57:171-92. [Crossref] [PubMed]
- Desmedt C, Fumagalli D, Pietri E, et al. Uncovering the genomic heterogeneity of multifocal breast cancer. J Pathol 2015;236:457-66. [Crossref] [PubMed]
- Lebya K, Garcia-Smith R, Swaminathan R, et al. Towards a personalized surgical margin for breast conserving surgery-Implications of field cancerization in local recurrence. J Surg Oncol 2017;115:109-15. [Crossref] [PubMed]
- Edgerton ME, Chuang YL, Macklin P, et al. A novel, patient-specific mathematical pathology approach for assessment of surgical volume: application to ductal carcinoma in situ of the breast. Anal Cell Pathol (Amst) 2011;34:247-63. [Crossref] [PubMed]
- Mitchell SD. A step-by-step oncoplastic breast conservation surgical atlas of reproducible dissection techniques and anatomically ideal incision placement. Breast Cancer Res Treat 2017;165:505-16. [Crossref] [PubMed]
- Mattingly AE, Ma Z, Smith PD, et al. Early Postoperative Complications after Oncoplastic Reduction. South Med J 2017;110:660-6. [Crossref] [PubMed]
- Weber WP, Soysal SD, Zeindler J, et al. Current standards in oncoplastic breast conserving surgery. Breast 2017;34:S78-81. [Crossref] [PubMed]
- Tan MP. A Novel Segment Classification for Multifocal and Multicentric Breast Cancer to Facilitate Breast-Conservation Treatment. Breast J 2015;21:410-7. [Crossref] [PubMed]
- Tan M, Ung O. Alternative approaches for oncoplastic breast surgery. Ann Surg Oncol 2011;18:297-9. [Crossref] [PubMed]
- Tan MP, Ong EM, Amy D, et al. Integrating anatomy, radiology, pathology, and surgery: An alternative approach in resecting multifocal and multicentric breast carcinoma. Breast J 2017;23:663-9. [Crossref] [PubMed]
- Tan MP, Sitoh NY, Sitoh YY. Perspectives of Cosmesis following Breast Conservation for Multifocal and Multicentric Breast Cancers. Int J Breast Cancer 2015;2015:126793. [Crossref] [PubMed]
- Ataseven B, Lederer B, Blohmer JU, et al. Impact of multifocal or multicentric disease on surgery and locoregional, distant and overall survival of 6,134 breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 2015;22:1118-27. [Crossref] [PubMed]
- Newman LA. Role of Preoperative MRI in the Management of Newly Diagnosed Breast Cancer Patients. J Am Coll Surg 2020;230:331-9. [Crossref] [PubMed]
- MacNeill F, Karakatsanis A. Over surgery in breast cancer. Breast 2017;31:284-9. [Crossref] [PubMed]
- Petruolo O, Sevilimedu V, Montagna G, et al. How Often Does Modern Neoadjuvant Chemotherapy Downstage Patients to Breast-Conserving Surgery? Ann Surg Oncol 2021;28:287-94. [Crossref] [PubMed]
- Simons JM, Jacobs JG, Roijers JP, et al. Disease-free and overall survival after neoadjuvant chemotherapy in breast cancer: breast-conserving surgery compared to mastectomy in a large single-centre cohort study. Breast Cancer Res Treat 2021;185:441-51. [Crossref] [PubMed]
- Gadaleta E, Thorn GJ, Ross-Adams H, et al. Field cancerization in breast cancer. J Pathol 2022;257:561-74. [Crossref] [PubMed]
- Dushyanthen S, Beavis PA, Savas P, et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med 2015;13:202. [Crossref] [PubMed]
- Laas E, El Beheiry M, Masson JB, et al. Partial breast resection for multifocal lower quadrant breast tumour using virtual reality. BMJ Case Rep 2021;14:e241608. [Crossref] [PubMed]
- Ingram J, Beasant L, Benson J, et al. The challenge of equipoise: qualitative interviews exploring the views of health professionals and women with multiple ipsilateral breast cancer on recruitment to a surgical randomised controlled feasibility trial. Pilot Feasibility Stud 2022;8:46. [Crossref] [PubMed]


