
Alternative donor sites in autologous breast reconstruction: a clinical practice review of the PAP flap
Introduction
Background
Abdominally-based free flaps have become the gold standard for autologous breast reconstruction (1-3). However, for some patients, abdominally-based flaps may be inadequate or otherwise contraindicated for breast reconstruction; other donor sites should be considered in these situations. Given their robust and reliable blood supply, thigh-based flaps were historically used as workhorse flaps for a variety of defects. The profunda artery perforator (PAP) flap is one such flap that emerged from an advancement of knowledge regarding thigh soft tissue anatomy and vascular supply, expanding is utility into the world of breast reconstruction (4).
In the early 1980’s, Hurteau et al. described ischial ulcer reconstruction using a posterior thigh based V-Y advancement flap supplied by perforators from the profunda artery (5). A couple years later, Lee described a lateral thigh free flap based on the third perforating vessel of the profunda artery (6). In 1984, Song et al. (7) published a series describing the anterolateral, anteromedial, and posterior thigh flaps. They described the vascular pedicles of the thigh based flaps as longer, larger in caliber, and more reliable than many of the other flaps available at the time (7). In 2001, Angrigiani et al. (8) published a case series of 20 cadaver dissections and 25 flaps, both pedicled and free. They described an adductor flap with a vascular supply originating from the first cutaneous perforator off the profunda artery that pierces through the adductor magnus to supply a large skin and soft tissue territory. In their study, they found an average pedicle length of 7–9 cm, and average vessel diameter of 2 mm (8). This adductor flap has since evolved into what is now known as the PAP flap.
In 2010, Allen went on to perform the first live PAP flap for breast reconstruction in Mexico City (9). In 2012, he published a series on his surgical technique and outcomes for breast reconstruction (10). Since then, the PAP flap has gained traction as a popular alternative option for autologous breast reconstruction when abdominally-based flaps are unavailable.
Rationale and knowledge gap
Breast reconstruction has become increasingly prevalent within plastic surgery, with procedure volume nearly doubling within the last 20 years (11). Between the two main reconstructive options, autologous reconstruction offers many benefits over implant-based reconstruction. Notably, it avoids the use of permanent prostheses that may be subject to capsular contracture, implant rupture, and provides a more natural-looking breast that ptoses over time (12,13). The field of microsurgery has also allowed for tremendous advances in autologous breast reconstruction. Refinements in surgical technique and patient management have allowed for decreased operative times, reduced morbidity, quicker recovery, and improved clinical and patient reported outcomes for those undergoing autologous breast reconstruction (13-15). While abdominal free flaps based on the deep inferior epigastric vessels remain the gold standard for autologous breast reconstruction, the PAP flap can provide a good alternative option for microsurgeons to have in their reconstructive armamentariums.
Objective
With the prevalence of autologous breast reconstruction seen today, a review of the current knowledge surrounding the PAP flap is pertinent. This clinical practice review will summarize and consolidate the anatomy, characteristics, and surgical technique involved in utilizing the PAP flap in the context of breast reconstruction, as well as provide a review of current literature discussing flap and patient outcomes.
Relevant anatomy
The posterior thigh is loosely defined as the region bounded superiorly by the gluteal fold, inferiorly by the popliteal fossa, medially by the adductors, and laterally by the iliotibial band. The posterior thigh compartment includes the biceps femoris, semitendinosus, and semimembranosus, which are supplied the profunda femora artery (16).
As the external iliac artery courses distal to the inguinal ligament, it becomes the common femoral artery. The common femoral artery splits into two main branches about 1–4 cm distal to the inguinal ligament. The common femoral artery continues superficially in the thigh and gives no major branches in the thigh (16). The profunda artery (deep femoral artery) travels posterolaterally between the adductor longus and pectineus muscles to enter the posterior thigh compartment. The first branches of the profunda are the medial and lateral circumflex femoral arteries. As the vessel travels distally, it usually gives off three perforating branches laterally, before terminating as a fourth perforating vessel. These large perforating vessels give off smaller, interconnected branches that form collaterals within the deep system (17). The first of these main perforating branches supplies the adductor muscles and the gracilis. The second and third of the perforating branches supply the biceps femoris, semimembranosus, and vastus lateralis. Each perforating branch also sends off musculocutaneous and/or septocutaneous perforators to supply the overlying skin in the posterior thigh region (16,17).
The profunda artery gives off at least 2–5 musculocutaneous or septocutaneous perforators that supply the medial posterior thigh skin. Perforators can be found on a line extending from the ischium to the lateral femoral condyle, and the first skin perforator is generally found within 8 cm of the inferior gluteal crease (IGC) (16,18-20). Average pedicle length is 6.8–11 cm and perforator diameter is at least 1.9 mm (16,19-21).
PAP flap: advantages and disadvantages
Today, the deep inferior epigastric perforator (DIEP) flap is the flap of choice for patients undergoing autologous breast reconstruction (12). However, some patients are deemed to be poor candidates for abdominally-based autologous reconstruction. This includes those with prior abdominoplasties or abdominally-based flaps, extensive abdominal surgery, thin patients with minimal abdominal soft tissue, women who desire future pregnancies, or patients who do not want large abdominal scars.
In patients who are not candidates for abdominally-based autologous reconstruction, thigh-based flaps are a favorable alternative. The PAP flap has become an increasingly popular option over gracilis-based flaps such as the transverse upper gracilis (TUG), diagonal upper gracilis (DUG), and vertical upper gracilis (VUG) for breast reconstruction because of its muscle-sparing nature. Leaving the thigh musculature intact reduces donor site pain and morbidity, as well as minimizes dead space and seroma formation postoperatively (22,23). The PAP flap can also provide larger flap volumes, a longer and larger pedicle, and larger skin paddles for skin resurfacing when needed. In a systematic review by Jo et al., the PAP flap was found to have longer pedicle length, higher flap weight, less donor site wound dehiscence, and comparable rates of flap loss and fat necrosis compared to the TUG flap (24). Another benefit of the PAP flap over gracilis myocutaneous flaps is the former’s further posterior zone of dissection and flap elevation, which helps avoid disruption of major lymphatic drainage of the lower extremity and thus reduces the risk of postoperative lymphedema (23,25).
Compared to gluteal flaps for breast reconstruction, PAP flap scars can be hidden within the gluteal crease and does not sacrifice gluteal contour. Upper medial thigh tissue found in PAP flaps is more malleable than gluteal and abdominal soft tissue, making it easier to shape and cone into a natural looking breast (12).
While the concept of neurotization of flaps for sensate breast reconstruction remains controversial, sensory nerves in the thigh have been described as permissive for harvest and coaptation and neurotization of PAP flaps has been described (26,27). The first successful neurotized PAP was published by Dayan and Allen, by anastomosing the anterior branch of the obturator nerve to the lateral branch of the T4 intercostal nerve (27). In a cadaveric study, Song et al. (28) proposed a neurotized PAP using the posterior femoral cutaneous nerve as a potential donor nerve.
The main disadvantage of PAP flap reconstruction of the breast remains its relatively limited volume for large reconstructions, especially compared to the DIEP flap (12,29). Average DIEP flap volumes have been reported to be around 700 g, while PAP flap volumes average 220 to 405 g with the ability to expand to 480 g by modifying flap design (29-33). Augmenting with another flap, implant, or fat grafting represent additional potential workarounds to this drawback.
Flap design and considerations
The first described PAP flaps were oriented transversely (tPAP), with tissue taken as a crescent shaped flap based on a proximal PAP high up in the thigh. This allowed for scars to be well hidden within the IGC, but flap width was limited to 6–8 cm to allow for primary closure without excessive tension (27,34). While the transversely designed scar is more easily concealed, it is also placed under tension when the patient is sitting, increasing the risk of delayed healing or wound complications. Furthermore, it may cause paresthesia if the posterior cutaneous nerve is injured (27).
The vertical PAP (vPAP) modified the skin paddle orientation to allow for dissection along a wider front to preferentially select the largest perforator along the profunda—not necessarily the most proximal perforator. The vertical skin paddle allows for the scar to be hidden within the medial thigh similar to that of a vertical thighplasty incision, without the concern for pressure on the incision while sitting (35,36).
The “fleur-de-lis” modification of the PAP flap (the “fleur-de-PAP”) allows for a flap that incorporates almost all, if not all, of the PAP angiosome, increasing soft tissue yield from a single donor site to meet the needs of a larger volume reconstruction (29,37,38). However, because the fleur-de-PAP combines both vertical and transverse skin paddle designs, the downsides of both are conferred to the donor site.
The senior author first introduced the diagonal PAP (dPAP) flap design in 2019 (27). In this modification, the skin paddle is oriented diagonally along the resting skin tension lines, thus allowing for a larger, wider skin paddle that can still be closed with minimal tension and reduced risk of wound dehiscence. The dPAP, like the vPAP, also avoids leaving scars over pressure points while patients are in the seated position. These advantages conferred by the dPAP has allowed it to become our preferred skin paddle design for PAP flaps.
We generally prefer the ipsilateral thigh for breast reconstruction, although contralateral reconstruction is also a viable option, given the central location and single perforator in most PAP flaps. If one PAP flap lacks sufficient volume for breast reconstruction, stacked PAP flaps are a good option (21,39). The PAP flap can also serve to augment breast reconstruction in patients undergoing DIEP flap reconstruction. Bilateral stacked DIEPs and PAPs, also known as four-flap reconstructions, have been successfully performed with great success when a single donor site does not provide adequate volume for bilateral reconstruction (40,41).
Surgical technique
Preoperative imaging
Preoperative imaging has become routine for surgical planning before free flap surgery. With many noninvasive advanced imaging modalities readily available, computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are increasingly used to assess perforator size, location, and anatomic variants, given their high degree of accuracy in predicting perforator anatomy in situ (Figure 1A) (19). This is particularly helpful for tPAP flaps when trying to ensure that a dominant perforator is located within 5–6 cm of the IGC. In instances where there is no dominant perforator close enough to the IGC, a dPAP flap can be planned and discussed with the patient.
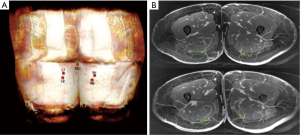
We prefer MRA studies for its three-dimensional reprocessing techniques and ability to provide high-definition perforator anatomy. The surgeon uses imaging to identify the dominant perforator(s) and map their intramuscular course, as well as measuring overall perforator length (Figure 1B). This allows for concordant planning of skin paddle location and placement of incisions.
All procedures performed in this work were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Preoperative marking
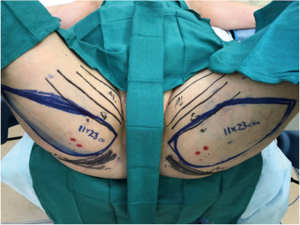
The surgeon’s preferred skin paddle orientation, as stated before, is the dPAP, and thus markings for this are described. The patient is marked standing up, with confirmation of markings when the patient is supine on the operating room (OR) table. The adductor longus, gracilis, IGC, and midline medial thigh are marked out. The predicted location of the dominant perforator seen on MRA is marked out, and later confirmed with Doppler signal in the OR. The anterior incision is marked starting along the posterior border of the gracilis, curving posteriorly starting approximately 8 cm inferior to the IGC. The posterior incision is estimated by performing a pinch test along Langer’s lines to see what can be closed under minimal tension. The posterior incision is then marked out, creating an ellipse, making sure to include the PAP perforator within the skin paddle (Figure 2).
Operative technique
After the patient is anesthetized and intubated, a foley is placed and the patient is positioned in lithotomy. A split leg bed or leaving the patient frog legged are also viable options, although in our experience the split leg bed and frog leg positioning are bulkier and more cumbersome to work around than the lithotomy position. All three options allow for a two-team approach such that dissection in the chest and lower extremity can be performed simultaneously.
Once the patient is positioned, prepped, and draped, the anterior incision is made. Dissection is carried out with electrocautery down to gracilis muscle. The investing fascia of the gracilis is incised, and dissection is carried out along the superficial surface of the gracilis, retracting the muscle anterolaterally. Next, the adductor magnus muscle is identified and its fascia is likewise incised. Dissection proceeds posterolaterally along the adductor magnus until perforators arising through the muscle into the skin are identified. It is important to use the entire length of the anterior incision while dissecting to avoid working in a narrow tunnel that limits visualization and increases the risk of avulsing or otherwise injuring perforators.
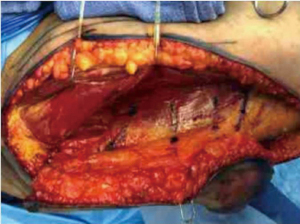
Once perforator(s) are identified, they are dissected retrograde using bipolar cautery until their source vessel, the profunda artery, is reached, or until adequate perforator length and vessel caliber are attained (Figure 3). The perforator is then divided, and if the flap is ready to be harvested, the posterior incision is made. If the flap is not yet ready to be harvested at this point, the flap can remain attached posteriorly and will receive adequate perfusion via posterior perforators until the flap is ready for transfer up to the chest. When making the posterior incision, the subcutaneous tissue can be beveled out to increase flap volume if needed.
In the chest, microsurgical anastomosis is performed to the anterograde internal mammary artery and vein when available. If not available for anastomosis or for cases utilizing stacked flaps, the retrograde internal mammary vessels or thoracodorsal vessels are also commonly used (42-45). After successful anastomosis, SPY-PHY (Stryker Corp., Kalamazoo, MI, USA) fluorescence imaging is used to assess flap perfusion. Any tissue with poor or questionable perfusion is excised, and the flap is then inset using absorbable sutures. Care should be taken to ensure sufficient inferior and medial pole fullness, as these areas tend to be difficult to augment during later revisional procedures (12). The flap is deepithelialized around a monitoring skin paddle, and the breast is closed over a closed suction drain sitting far from the anastomosis.
Closure of the donor site requires selective posterior skin flap elevation off muscle fascia to help reduce tension and aid in robust closure. Care should be taken to undermine just enough skin to be able to close the wound with minimal tension to avoid undue dissection. A multilayered closure with quilting sutures over a surgical drain help to reduce the chance of seroma formation. To reduce postoperative pain from the donor site, we inject liposomal bupivacaine into the deep and superficial soft tissues before closure.
Postoperative care
Lower extremity compression garments are applied in the immediate post-operative period and continued for 3 weeks after surgery to improve postoperative contour and reduce seroma formation and scar hypertrophy or widening. Patients are instructed to refrain from strenuous physical activity for at least 6 weeks after surgery, at which point restrictions may slowly be liberalized, as deemed appropriate.
Enhanced recovery after surgery (ERAS) protocols developed for abdominally-based autologous breast reconstruction have been shown to improve patient recovery and reduce opioid usage (46). These protocols have also been adapted for PAP flaps, and have similarly been shown to decrease operative time, length of stay, and opioid usage (47).
Postoperative flap monitoring is typically performed with pencil Doppler throughout the inpatient stay. Patients are mobilized out of bed on postoperative day 1, and foley catheters are removed on either postoperative day 0 or 1, if the surgery was unilateral or bilateral, respectively. With our current ERAS protocol, patients are generally ready for discharge on postoperative day 2 for unilateral flaps, and day 3 for bilateral flaps. Patients are educated on drain care and incision care at home, as well as advised on clinical flap monitoring before discharge.
Outcomes
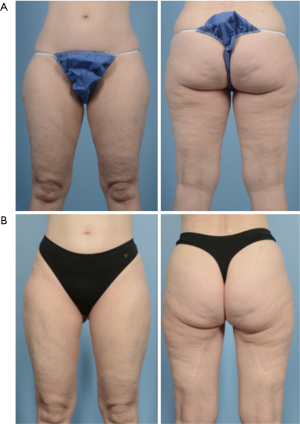
Overall success rate of PAP flaps is high both at our institution as well as in the literature, with published flap success rates near 97–100% (10,30,31,48-50). The majority of complications after PAP flap reconstruction are related to the donor site, and return to the OR is uncommon. In 2012, Allen et al. published their early series of 27 PAP flaps, with 100% flap success rate and two flaps that developed less than 10% fat necrosis postoperatively. They had two donor site complications, including one patient with seroma and another with hematoma that were treated with drain and local wound care, respectively (10). In 2016, the group published their updated results from 164 flaps, reporting over 99% flap success rate with only one flap loss. Complications included fat necrosis (7%), seroma (6%), donor-site wound dehiscence (3.6%), and hematoma (1.9%). Of note, they did not have any patients who developed postoperative lymphedema (30). In 2017, Haddock et al. published a series of their first 101 PAP flaps, with 2 total flaps losses (2.0%), 1 partial flap loss (1.0%), 11 (10.9%) patients with donor site wound dehiscence, and 6 (5.9%) patients with donor site cellulitis (49). In 2020, the group published an updated series including 265 PAP flaps. They noted 3% total flap loss, with the remaining complications related to donor sites: 6.8% developed wounds requiring debridement or negative pressure wound therapy, 4.9% developed infections, 4.5% had seromas, and 2.6% had hematomas. They also noted a 1.4% rate of deep vein thrombosis or pulmonary embolism (31). A meta analysis of 516 PAP flaps showed an overall flap success rate of 99%, and overall complication rate of 23%. Wound dehiscence was found to be the most common complication at 6%, and seroma (2%) and hematoma (1%) were relatively uncommon (50). In 2020, Cho et al. published a classification of donor site wounds after PAP flap reconstruction, and noted that wound dehiscence of the medial thigh is correlated to increasing body mass index (BMI) (51). Given the overall high success rate and low complication rate, patient reported outcomes after breast reconstruction using the BREAST-Q questionnaire have also shown high patient satisfaction (Figures 4,5) (31,48).

Strengths and limitations
This review aims to provide a thorough and in-depth overview of the PAP flap and its applications relevant to breast reconstruction. The clinical pearls provided by the senior author’s preferences may allow other microsurgeons a quicker learning curve and facilitate efficient dissection in the OR. Shortcomings of this review are mainly related to the relatively newer development of this flap and the limited data on longer term outcomes, especially across large patient populations. Another limitation of this review includes the fact that certain aspects such as preoperative markings, patient positioning, and imaging techniques are more anecdotal in nature based on the senior author’s preferences and expert opinion. Further studies are encouraged to further elucidate long term outcomes for patients undergoing PAP flap reconstruction.
Conclusions
The prevalence of autologous breast reconstruction necessitates a thorough understanding on the part of the plastic surgeon of all the reconstructive options at his/her disposal. While the DIEP flap has become the workhorse in this regard, the PAP flap represents a strong alternative or adjunct for patients in which abdominally-based flaps are inadequate or insufficient. With proper planning and surgical technique, its anatomy and physical characteristics allow the PAP flap to be utilized as a versatile tool for the purpose of breast reconstruction. Most importantly, it achieves high reconstructive success rates and patient satisfaction ratings while minimizing donor site morbidity in the thigh. Indeed, both patients and surgeons can find consolation that the PAP flap does not sacrifice much in way of fulfilling the needs of breast reconstruction.
Acknowledgments
The authors would like to acknowledge Dr. Martin Prince of Weil-Cornell Imaging for giving permission to use the images seen in Figure 1A,1B.
Funding: This work was supported in part through the National Institutes of Health/National Cancer Institute Cancer Center (support grant P30 CA008748), which supports Memorial Sloan Kettering Cancer Center’s research infrastructure.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ketan M. Patel and Ara A. Salibian) for the series “Advances in Microsurgical Breast Reconstruction” published in Gland Surgery. The article has undergone external peer review.
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-603/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-603/coif). The series “Advances in Microsurgical Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this work were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Cancer Research Fund International. Breast cancer statistics. Available online: https://www.wcrf.org/cancer-trends/breast-cancer-statistics/
- Tachi M, Yamada A. Choice of flaps for breast reconstruction. Int J Clin Oncol 2005;10:289-97. [Crossref] [PubMed]
- Pien I, Caccavale S, Cheung MC, et al. Evolving Trends in Autologous Breast Reconstruction: Is the Deep Inferior Epigastric Artery Perforator Flap Taking Over? Ann Plast Surg 2016;76:489-93. [Crossref] [PubMed]
- Gurunluoglu R, Gurunluoglu A, Williams SA, et al. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg 2013;70:103-10. [Crossref] [PubMed]
- Hurteau JE, Bostwick J, Nahai F, et al. V-Y advancement of hamstring musculocuataneous flap for coverage of ischial pressure sores. Plast Reconstr Surg 1981;68:539-42. [Crossref] [PubMed]
- Lee HY, Na SY, Son YM, et al. A malignant melanoma associated with a blue nevus of the lip. Ann Dermatol 2010;22:119-24. [Crossref] [PubMed]
- Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg 1984;37:149-59. [Crossref] [PubMed]
- Angrigiani C, Grilli D, Thorne CH. The adductor flap: a new method for transferring posterior and medial thigh skin. Plast Reconstr Surg 2001;107:1725-31. [Crossref] [PubMed]
- Allen RJ. Live surgery performed. In: 13th International Course on Perforator Flaps, Mexico City, 2010.
- Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg 2012;129:16e-23e. [Crossref] [PubMed]
- American Society of Plastic Surgeons. Plastic surgery statistics report 2020. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf
- Allen RJ, Mehrara BJ. Breast Reconstruction. Plastic Surgery - Principles and Practice. Elsevier Inc., 2021:535-64.
- Nelson JA, Allen RJ Jr, Polanco T, et al. Long-term Patient-reported Outcomes Following Postmastectomy Breast Reconstruction: An 8-year Examination of 3268 Patients. Ann Surg 2019;270:473-83. [Crossref] [PubMed]
- Bonde C, Khorasani H, Eriksen K, et al. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: A case control study. J Plast Surg Hand Surg 2015;49:367-71. [Crossref] [PubMed]
- Rozen WM, Ashton MW. Improving outcomes in autologous breast reconstruction. Aesthetic Plast Surg 2009;33:327-35. [Crossref] [PubMed]
- Ahmadzadeh R, Bergeron L, Tang M, et al. The posterior thigh perforator flap or profunda femoris artery perforator flap. Plast Reconstr Surg 2007;119:194-200. [Crossref] [PubMed]
- Rubin JA, Whetzel TP, Stevenson TR. The posterior thigh fasciocutaneous flap: vascular anatomy and clinical application. Plast Reconstr Surg 1995;95:1228-39. [Crossref] [PubMed]
- Saad A, Sadeghi A, Allen RJ. The anatomic basis of the profunda femoris artery perforator flap: a new option for autologous breast reconstruction--a cadaveric and computer tomography angiogram study. J Reconstr Microsurg 2012;28:381-6. [Crossref] [PubMed]
- Haddock NT, Greaney P, Otterburn D, et al. Predicting perforator location on preoperative imaging for the profunda artery perforator flap. Microsurgery 2012;32:507-11. [Crossref] [PubMed]
- DeLong MR, Hughes DB, Bond JE, et al. A detailed evaluation of the anatomical variations of the profunda artery perforator flap using computed tomographic angiograms. Plast Reconstr Surg 2014;134:186e-92e. [Crossref] [PubMed]
- Haddock NT, Cho MJ, Gassman A, et al. Stacked Profunda Artery Perforator Flap for Breast Reconstruction in Failed or Unavailable Deep Inferior Epigastric Perforator Flap. Plast Reconstr Surg 2019;143:488e-94e. [Crossref] [PubMed]
- Park JE, Alkureishi LWT, Song DH. TUGs into VUGs and Friendly BUGs: Transforming the Gracilis Territory into the Best Secondary Breast Reconstructive Option. Plast Reconstr Surg 2015;136:447-54. [Crossref] [PubMed]
- Dayan JH, Allen RJ Jr. Lower Extremity Free Flaps for Breast Reconstruction. Plast Reconstr Surg 2017;140:77S-86S. [Crossref] [PubMed]
- Jo T, Kim EK, Eom JS, et al. Comparison of transverse upper gracilis and profunda femoris artery perforator flaps for breast reconstruction: A systematic review. Microsurgery 2020;40:916-28. [Crossref] [PubMed]
- Hunter JE, Lardi AM, Dower DR, et al. Evolution from the TUG to PAP flap for breast reconstruction: Comparison and refinements of technique. J Plast Reconstr Aesthet Surg 2015;68:960-5. [Crossref] [PubMed]
- Yano T, Karakawa R, Yoshimatsu H, et al. The Feasibility of Harvesting an Innervated Profunda Artery Perforator Flap for Breast Reconstruction. Plast Reconstr Surg Glob Open 2020;8:e3160. [Crossref] [PubMed]
- Dayan JH, Allen RJ Jr. Neurotized Diagonal Profunda Artery Perforator Flaps for Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2463. [Crossref] [PubMed]
- Song B, Kumbla PA, Boyd C, et al. The Feasibility of a Sensate Profunda Artery Perforator Flap in Autologous Breast Reconstruction: An Anatomic Study for Clinical Application. Ann Plast Surg 2020;84:S451-4. [Crossref] [PubMed]
- Hunsinger V, Lhuaire M, Haddad K, et al. Medium- and Large-Sized Autologous Breast Reconstruction using a Fleur-de-lys Profunda Femoris Artery Perforator Flap Design: A Report Comparing Results with the Horizontal Profunda Femoris Artery Perforator Flap. J Reconstr Microsurg 2019;35:8-14. [Crossref] [PubMed]
- Allen RJ Jr, Lee ZH, Mayo JL, et al. The Profunda Artery Perforator Flap Experience for Breast Reconstruction. Plast Reconstr Surg 2016;138:968-75. [Crossref] [PubMed]
- Haddock NT, Teotia SS. Consecutive 265 Profunda Artery Perforator Flaps: Refinements, Satisfaction, and Functional Outcomes. Plast Reconstr Surg Glob Open 2020;8:e2682. [Crossref] [PubMed]
- Woo KJ, Mun GH. Estimation of DIEP flap weight for breast reconstruction by the pinch test. Microsurgery 2017;37:786-92. [Crossref] [PubMed]
- Eder M, Raith S, Jalali J, et al. Three-dimensional prediction of free-flap volume in autologous breast reconstruction by CT angiography imaging. Int J Comput Assist Radiol Surg 2014;9:541-9. [Crossref] [PubMed]
- Paletta C, Bartell T, Shehadi S. Applications of the posterior thigh flap. Ann Plast Surg 1993;30:41-7. [Crossref] [PubMed]
- Rivera-Serrano CM, Aljaaly HA, Wu J, et al. Vertical PAP Flap: Simultaneous Longitudinal Profunda Artery Perforator Flaps for Bilateral Breast Reconstructions. Plast Reconstr Surg Glob Open 2017;5:e1189. [Crossref] [PubMed]
- Scaglioni MF, Chen YC, Lindenblatt N, et al. The vertical posteromedial thigh (vPMT) flap for autologous breast reconstruction: A novel flap design. Microsurgery 2017;37:371-6. [Crossref] [PubMed]
- Saussy K, Stalder MW, Delatte SJ, et al. The fleur-de-PAP flap for bilateral breast reconstruction. J Reconstr Microsurg Open 2017;2:e1-3. [Crossref]
- Bourn L, Torabi R, Stalder MW, et al. Mosaic Fleur-de-Profunda Artery Perforator Flap for Autologous Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2166. [Crossref] [PubMed]
- Blechman KM, Broer PN, Tanna N, et al. Stacked profunda artery perforator flaps for unilateral breast reconstruction: a case report. J Reconstr Microsurg 2013;29:631-4. [Crossref] [PubMed]
- Haddock NT, Cho MJ, Teotia SS. Comparative Analysis of Single versus Stacked Free Flap Breast Reconstruction: A Single-Center Experience. Plast Reconstr Surg 2019;144:369e-77e. [Crossref] [PubMed]
- Haddock NT, Suszynski TM, Teotia SS. Consecutive Bilateral Breast Reconstruction Using Stacked Abdominally Based and Posterior Thigh Free Flaps. Plast Reconstr Surg 2021;147:294-303. [Crossref] [PubMed]
- Teotia SS, Dumestre DO, Jayaraman AP, et al. Revisiting Anastomosis to the Retrograde Internal Mammary System in Stacked Free Flap Breast Reconstruction: An Algorithmic Approach to Recipient-Site Selection. Plast Reconstr Surg 2020;145:880-7. [Crossref] [PubMed]
- Teotia SS, Cho MJ, Haddock NT. Salvaging Breast Reconstruction: Profunda Artery Perforator Flaps Using Thoracodorsal Vessels. Plast Reconstr Surg Glob Open 2018;6:e1837. [Crossref] [PubMed]
- Stalder MW, Lam J, Allen RJ, et al. Using the Retrograde Internal Mammary System for Stacked Perforator Flap Breast Reconstruction: 71 Breast Reconstructions in 53 Consecutive Patients. Plast Reconstr Surg 2016;137:265e-77e. [Crossref] [PubMed]
- Mayo JL, Canizares O, Torabi R, et al. Expanding the Applications of the Profunda Artery Perforator Flap. Plast Reconstr Surg 2016;137:663-9. [Crossref] [PubMed]
- Temple-Oberle C, Shea-Budgell MA, Tan M, et al. Consensus Review of Optimal Perioperative Care in Breast Reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast Reconstr Surg 2017;139:1056e-71e. [Crossref] [PubMed]
- Cho MJ, Garza R, Teotia SS, et al. Utility of ERAS Pathway in Nonabdominal-Based Microsurgical Breast Reconstruction: Efficacy in PAP Flap Reconstruction? J Reconstr Microsurg 2022;38:371-7. [Crossref] [PubMed]
- Atzeni M, Salzillo R, Haywood R, et al. Breast reconstruction using the profunda artery perforator (PAP) flap: Technical refinements and evolution, outcomes, and patient satisfaction based on 116 consecutive flaps. J Plast Reconstr Aesthet Surg 2022;75:1617-24. [Crossref] [PubMed]
- Haddock NT, Gassman A, Cho MJ, et al. 101 Consecutive Profunda Artery Perforator Flaps in Breast Reconstruction: Lessons Learned with Our Early Experience. Plast Reconstr Surg 2017;140:229-39. [Crossref] [PubMed]
- Qian B, Xiong L, Li J, et al. A Systematic Review and Meta-Analysis on Microsurgical Safety and Efficacy of Profunda Artery Perforator Flap in Breast Reconstruction. J Oncol 2019;2019:9506720. [Crossref] [PubMed]
- Cho MJ, Teotia SS, Haddock NT. Classification and Management of Donor-Site Wound Complications in the Profunda Artery Perforator Flap for Breast Reconstruction. J Reconstr Microsurg 2020;36:110-5. [Crossref] [PubMed]


