
Different biopsy techniques for different pancreas graft locations after homolateral simultaneous pancreas-kidney transplantation
Highlight box
Key findings
• Pancreatic graft biopsy is safe and feasible after simultaneous pancreas-kidney transplantation.
• Different biopsy strategies should be considered according to the anatomical location of the transplanted pancreas.
What is known and what is new?
• A few centers have reported on various ways of biopsying transplanted pancreatic tissue.
• Unlike in previous studies, we recommend that different biopsy methods are selected depending on the anatomical location of the transplanted pancreas.
What is the implication, and what should change now?
• There are various surgical methods for simultaneous pancreas-kidney transplantation, and the anatomical location of the transplanted pancreas also differs, so different biopsy methods should be used.
Introduction
Simultaneous pancreas-kidney (SPK) transplantation is currently the most effective method for the treatment of diabetes complicated with stage 5 chronic kidney disease (1). Postoperative increases in serum amylase and lipase concentrations are common, and differential diagnosis is difficult, with the only way to confirm the diagnosis being pancreatic biopsy (2).
Because the transplanted pancreas is located in the abdominal cavity, surrounded by the intestinal tract, and often accompanied by adhesions, the risks associated with biopsy are high (3). However, there are few reports regarding the selection of different biopsy methods. In this paper, we summarize our experiences with pancreatic biopsy after SPK transplantation in 4 patients. We present the following article in accordance with the MDAR and AME Case Series reporting checklists (available at https://gs.amegroups.com/article/view/10.21037/gs-22-414/rc).
Methods
Baseline patient data
Between September 2016 and December 2021, 235 homolateral SPK transplantations were performed at the Department of Organ Transplantation, The Second Affiliated Hospital of Guangzhou Medical University. All organs were from deceased donors (citizens). Elevated serum amylase and lipase concentrations were observed in many recipients. A biopsy of the transplanted pancreas was performed in 4 of these patients after they provided written informed consent. Pathologists followed the Banff criteria to determine whether there was rejection of the graft (4). Baseline data for the 4 patients are presented in Table 1.
Table 1
| Patient no. | Sex | Age (years) | BMI (kg/m2) | Primary disease and comorbidity | Immunosuppressive maintenance regimen | Time of biopsy after transplantation (months) | Reason for biopsy |
|---|---|---|---|---|---|---|---|
| 1 | Female | 31 | 20.6 | DN△ | FK506 + MPA | 29 | Elevated serum AMS and LPS |
| Type 1 diabetes | |||||||
| Renal anemia | |||||||
| 2 | Male | 66 | 26.1 | DN△ | FK506 + MPA + Pred | 22 | Elevated serum AMS and LPS |
| Type 2 diabetes | |||||||
| Hyperlipidemia | |||||||
| CHD | |||||||
| 3 | Male | 51 | 22.6 | DN△ | FK506 + MPA | 4 | Elevated serum AMS and LPS |
| Type 2 diabetes | |||||||
| Renal anemia | |||||||
| 4 | Male | 50 | 22.4 | DN△ | FK506 + MPA + Pred | 34 | Recurrent fever after treatment for PTLD |
| Type 2 diabetes | |||||||
| Fever | |||||||
| Renal anemia |
△, primary disease. BMI, body mass index; DN, diabetic nephropathy; FK506, tacrolimus; MPA, mycophenolate mofetil; AMS, amylase; LPS, lipase; CHD, coronary heart disease; Pred, prednisolone; PTLD, posttransplant lymphoproliferative disorder.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Institutional Review Board of the Second Affiliated Hospital of Guangzhou Medical University (number 2020-hs-43) and informed consent was taken from all individual participants.
Immunosuppressive protocols
Polyclonal and monoclonal antibodies were selected for induction therapy. Preoperatively, patients received an intravenous injection of methylprednisolone (MP; 250–500 mg), and on days 1, 2, and 3 postoperatively, patients were administered 500, 250, and 250 mg of MP, respectively. For maintenance therapy, all patients who underwent SPK transplantation received tacrolimus (FK506; 0.05–0.1 mg/kg per day), mycophenolate mofetil (MMF; 1–1.5 g/day), and steroids (prednisone). At 3 months after transplantation, the dose of FK506 was adjusted to a trough concentration of 6–8 ng/mL. The dose of prednisone was 30 mg p.o. on day 4 after surgery, with a reduction of 5 mg of prednisone every 7 days until a maintenance dose of 10 or 5 mg was reached. If the preoperative diagnosis of diabetic nephropathy was confirmed, prednisone was withdrawn.
Location of the transplanted pancreas and selection of biopsy methods
Homolateral SPK transplantation was performed in the Department of Organ Transplantation, The Second Affiliated Hospital of Guangzhou Medical University. A schematic diagram of SPK transplantation is presented in a our previously published paper (5). The transplanted pancreas was located in the right lower abdomen.
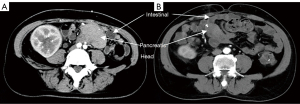
Before biopsy, computed tomography (CT) was used to ascertain the anatomical position of transplanted pancreas (Figure 1) and facilitate selection of the biopsy method.
Percutaneous CT combined with color Doppler-guided needle biopsy
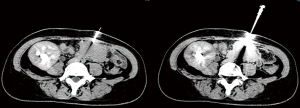
For percutaneous CT combined with color Doppler-guided needle biopsy, patients were supine in the CT interventional operating room. An enhanced CT scan was first performed to gain an understanding of the distribution of the artery of the head of the transplanted pancreas, with a location in the pancreatic head with sparse vascular distribution selected as the target puncture point. A needle was punctured through the abdominal wall to the pancreatic head under color Doppler imaging to visualize arteries and veins around the needlepoint and to avoid blood vessels as far as possible. Samples were obtained using an 18-G core biopsy needle as shown in Figure 2. Then, a gelatin sponge strip was inserted, and snake venom hemagglutinin was injected through the needle inlet channel to stop the bleeding. After withdrawal of the needle, pressure was applied for at least 20 min until there was no bleeding at the puncture point. Re-examination CT was used to confirm that there was no obvious effusion or blood around the puncture point.
Laparoscopic biopsy
Patients underwent a laparoscopic transplanted pancreas biopsy under general anesthesia in the operating room. Direct wedge biopsy or complementary needle biopsy was used. The pancreas body or tail was selected for direct wedge biopsy, and the biopsy site was sutured to stop the bleeding. A combined puncture biopsy was performed if necessary. The skin projection area of the head of the transplanted pancreas was taken as the insertion point, and the puncture biopsy was performed, with the intestinal tract and blood vessels being avoided under direct laparoscopic vision, as shown in Figure 3.

Postbiopsy treatment
After returning to the ward, the patients were monitored with electrocardiography for 24 h for any abdominal pain and other symptoms. In addition, hemoglobin, serum lipase, and serum amylase concentrations were monitored. The color of the drainage fluid was observed, and the volume of the drainage fluid was measured and tested for amylase and lipase for 3 consecutive days. The transplanted pancreas was examined with ultrasound for 3 consecutive days to detect any bleeding, pancreatic leakage, and other complications.
Statistical analysis
Data were analyzed using SPSS 22 (IBM Corporation). The Shapiro-Wilk test was used to evaluate the distribution of count data, while comparisons between groups were made using the Wilcoxon test. P<0.05 indicate a statistically significant difference.
Results
Histological findings and management
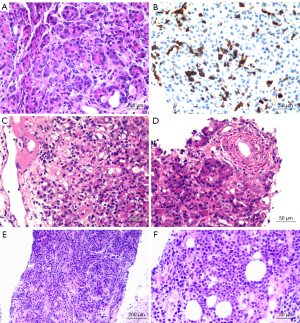
Pancreatic graft specimens were obtained from 4 patients using the techniques described in the Methods. Patient 3 (see Table 2) was not successfully sampled, with histological results indicating that the biopsy sample consisted of adipose tissue; the remaining patients were successfully sampled. Patient 1 experienced acute pancreatic rejection, patient 2 experienced pancreatitis, and Patient 4 experienced pancreatic plasmablastic lymphoma. Patients underwent appropriate treatment according to the pathological results (Table 2, Figure 4).
Table 2
| Patient no. | Biopsy way | Satisfactory specimen | Pathology results | Treatment |
|---|---|---|---|---|
| 1 | CT-guided puncture combined with color ultrasound | Yes | Multifocal acinar inflammation (predominantly CD8+ T cells), periacinar capillary vasculitis, interlobular septal inflammatory infiltration; predisposition to T cell-mediated rejection | MP + ATG (after histological report) |
| 2 | Laparoscopic wedge biopsy | Yes | Interlobular inflammatory infiltration, small phlebitis, ductitis, acinar inflammation, some acinar cell swelling, degeneration, necrosis; transplantation pancreatitis considered | (I) MP + ATG (before histological report) |
| (II) SS + PPI (before and after histological report) | ||||
| (III) FK506 changed to CsA after histological report | ||||
| 3 | Laparoscopic wedge biopsy | No | Adipose tissue, nerve tissue, and fibrous connective tissue; pancreatic tissue not seen | (I) SS + PPI |
| (II) MP + ATG + rituximab + PE + IVIG | ||||
| 4 | Laparoscopic wedge-shaped biopsy combined with needle biopsy | Yes | Lymphocytic cells diffuse in the pancreatic tissue, with medium to large size; some nuclear deviation consistent with plasmablastic lymphoma | (I) Rituximab + IVIG (diagnosis of PTLD and before histological report) |
| (II) MP + ATG + IVIG (before histological report) | ||||
| (III) Rituximab + lenalidomide (after histological report) |
CT, computed tomography; MP, methylprednisolone; ATG, anti-human T cell rabbit immunoglobulin; SS, somatostatin; PPI, proton pump inhibitor; FK506, tacrolimus; CsA, cyclosporin A; PE, plasma exchange; IVIG, intravenous immunoglobulin; PTLD, posttransplant lymphoproliferative disorder.
Treatment effect
At present, there are no uniform criteria for the success of antirejection treatment. Successful treatment will reduce serum lipase and amylase, but the rate and degree of decline differ across individual patients.
In the present series, lipase and amylase concentrations in patient 1 decreased to normal after antirejection therapy according to the pathological results. Prior to the biopsy, patient 2 was being treated for acute rejection, but the treatment effect was not satisfactory. After the biopsy, based on the pathological results, patient 2 underwent routine treatment for pancreatitis. In addition, FK506 was changed to cyclosporin A, after which lipase and amylase concentrations decreased to normal.
Because the biopsy was unsuccessful in patient 3, this patient was treated based on clinical judgment. Clinically, patient 3 was diagnosed with acute rejection of the transplanted pancreas and kidney. After antirejection therapy, amylase and lipase concentrations decreased, and the renal function of the transplanted kidney improved.
In patient 4, color ultrasound detected a space-occupying lesion in the head of the transplanted pancreas 2 months before biopsy; posttransplant lymphoproliferative disorder was subsequently diagnosed by positron emission tomography (PET) /CT and immunofixation electrophoresis. After rituximab plus intravenous immune globulin treatment, the patient’s body temperature returned to normal, but fever recurred several days later. Due to a lack of clear evidence for infection, treatment for acute rejection had poor effect. After the biopsy, pancreatic plasmablastic lymphoma was indicated, and the patient was treated with rituximab plus lenalidomide. However, the patient died 2 months later after the oncotherapy; the causes of death were cachexia, bone marrow suppression, and pulmonary infection.
The renal function of the transplants was stable before and after biopsy in all 4 patients. Changes in pancreas-related indicators before and after biopsy are presented in Table 3. The paired-sample Wilcoxon test was used to compare the indicators at stable stage, before biopsy, and after treatment, and there were no statistically significant differences (P>0.05).
Table 3
| Patient no. | Serum AMS (units/L) | Serum LPS (units/L) | FBG (mmol/L) | HbA1c (%) | Scr (µmol/L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stable period | Before biopsy | After treatment | Stable period | Before biopsy | After treatment | Stable period | Before biopsy | After treatment | Stable period | Before biopsy | After treatment | Stable period | Before biopsy | After treatment | |||||
| 1 | 60 | 1,078 | 123 | 47 | 2,119 | 145 | 4.9 | 5.6 | 4.8 | 6.0 | 5.9 | 6.0 | 74 | 77 | 68 | ||||
| 2 | 106 | 482 | 144 | 68 | 1,415 | 152 | 5.2 | 5.6 | 5.4 | 5.9 | 5.6 | 7.1 | 126 | 154 | 120 | ||||
| 3 | 110 | 1,470 | 402 | 58 | 933 | 738 | 5.7 | 5.8 | 6.4 | 4.8 | 5.0 | 5.6 | 127 | 159 | 132 | ||||
| 4 | 90 | 127 | 116 | 48 | 188 | 179 | 4.4 | 4.3 | 4.6 | 5.2 | 5.1 | 5.2 | 98 | 91 | 86 | ||||
| Median | 98 | 780 | 134 | 53 | 1,174 | 166 | 5.1 | 5.6 | 5.1 | 5.6 | 5.4 | 5.8 | 112 | 123 | 103 | ||||
LPS, lipase; FBG, fasting blood glucose; Hb, hemoglobin; Scr, serum creatinine.
Perioperative observations and follow-up
None of the 4 patients had perioperative complications, such as hemorrhage or pancreatic leakage. With the exception of patient 4, who died, the patients continue to be followed up and show normal pancreas transplantation status and renal function.
Discussion
Because the recipients of the SPK transplants were all diabetic patients with stage 5 chronic kidney disease, there was no need to remove their original pancreas, and both the transplanted pancreas and the recipient’s own pancreas were simultaneously present. Clinical diagnoses are often difficult in patients with elevated serum amylase and lipase concentrations, and problems such as pancreatitis of the transplanted pancreas, rejection of the transplanted pancreas (6,7), and pancreatitis of the original pancreas can occur. Differential diagnoses are difficult, and biopsy of the transplanted pancreas is the gold standard for diagnosis.
Biopsy of the transplanted pancreas has been difficult to perform in clinical practice (8,9). Compared with that of renal graft biopsy, the vascular distribution of the transplanted pancreas is complicated, and the pancreas is surrounded by the adherent recipient intestine. Moreover, if the puncture causes pancreatic or intestinal leakage, the consequences may be serious. Although some transplant centers have performed or are currently performing combined pancreas-kidney transplantation, transplanted pancreas biopsies are routinely performed only in a small number of transplant centers, and in different ways. At present, there are 5 main types of transplanted pancreas biopsy: percutaneous ultrasound-guided needle biopsy, CT-guided percutaneous puncture biopsy, laparoscopic biopsy, biopsy via a laparotomy, and endoscopic ultrasound-guided pancreas graft biopsy.
Percutaneous ultrasound-guided needle biopsy
In 2005, the University of Pittsburgh Medical Center reported a group of 50 patients who underwent combined pancreas–kidney transplantation, pancreas transplantation alone, and pancreas transplantation after kidney transplantation (10). In all, 120 percutaneous ultrasound-guided pancreatic puncture biopsies were performed, and the pancreatic head was initially selected as the puncture site. To avoid intestinal injury, the puncture site was later changed to the pancreatic body. The sampling success rate was 85% (n=102 times), and the failure rate was15% (n=18). The reported complications were hemorrhage in 1 patient, pancreatic leakage in 1 patient, and an elevation in pancreatic enzymes after puncture in 9 patients with no clinical symptoms of pancreatitis (10).
CT-guided percutaneous puncture biopsy
A 2015 paper reported a group of 17 SPK patients who underwent CT-guided percutaneous puncture biopsy because of elevated pancreatic enzymes (11). Complications included appendix perforation, subcutaneous hematoma, and sciatic nerve transient injury. Most of the pathological results were acute rejection (11).
In our case, the first technique we used was a combination of percutaneous ultrasound-guided needle biopsy and CT-guided percutaneous puncture biopsy, which had the advantages of convenient operation and little trauma to patients on the premise that CT suggested no intestinal wrapping between the transplanted pancreatic head and the anterior abdominal wall. The head of the pancreas was chosen as the puncture point because there were splenic arteries and splenic veins in the body and tail of the pancreas, for which bleeding could not be controlled easily, and there are 2 areas with little vascularity in the head of the pancreas. The first of these is above the head of the pancreas, between the anterior and posterior superior pancreaticoduodenal arteries, and the other is below the head of the pancreas, between the anterior and posterior inferior pancreaticoduodenal arteries. According to the homolateral SPK transplantation method, the latter area was placed toward the anterior abdominal wall, making it a more appropriate puncture point. The advantage of CT combined with ultrasound is that the best puncture point can be determined with CT angiography to avoid the artery (3), while the splenic vein can be avoided with use of color Doppler when the needle is inserted to the surface of the transplanted pancreatic head, which further ensures safety.
Laparoscopic biopsy
In 2017, Uva et al. reported a group of 95 combined pancreas-kidney transplantation recipients who underwent a total of 160 laparoscopic pancreas biopsies (89 diagnostic, 71 procedural) in Argentina (12). Kidney biopsies were performed with a 16-G needle puncture, and pancreas biopsies were performed with a wedge biopsy combined with a 16-G needle puncture. An open biopsy was performed in 4 patients due to the inability to obtain materials under laparoscopy. In 14 cases, there was too little pancreatic tissue, with some being mistaken for adipose and lymphatic tissue. Histological findings of the pancreas included 29 cases of acute cellular rejection, 1 case of acute fluid rejection, and 18 cases of nonrejection chronic disease, with normal pancreatic tissue being found in the remaining cases. There were 4 cases of intraoperative complications (2 cases each of duodenal contusion and renal hemorrhage). All complications were successfully treated by a small incision operation without pancreatic leakage or graft failure.
The advantages of a laparoscopic biopsy are that it is safe, reliable, and suitable for cases of intestinal wrapping around the pancreas head. Cases where the intestine is wrapped around the transplanted pancreas head are often accompanied by adhesions to varying degrees, and the coverage of the greater omentum makes it difficult to perform a wedge biopsy, so wedge sampling is mainly performed in the body and tail. If the adhesion around the body and tail of the pancreas is also serious, fine needle biopsy under the laparoscopic field of vision can be additionally applied, and the puncture site is still selected in the less vascular area of the pancreatic head. This was the situation in patient 4 in the present study, with laparoscopic operation in the process of mild separation having the potential to lead to bleeding because of adhesion. We could not confirm whether the tissue was peripancreatic fat or pancreas by wedge biopsy, so an auxiliary puncture biopsy under laparoscopic view was used. We were thus able to confirm that the wedge biopsy sample was fat tissue and the puncture biopsy sample was transplanted pancreas tissue.
In summary, although laparoscopic biopsy requires general anesthesia, the field of view is clear and bleeding at the biopsy site can be stopped using electrocoagulation or suture hemostasis, which is safer than percutaneous puncture biopsy.
Particular attention should be paid to the location of the visual and operation ports of the laparoscope. Because homolateral SPK transplantation is mainly located in the right lower abdomen, the right abdominal adhesion is more obvious than is the left, and the body and tail of the pancreas are the preferred biopsy sites, so we believe that it is more appropriate to use the left upper abdomen and left middle abdomen areas for visual and operation ports. In addition, we suggest that the surface projection area of the transplanted pancreas be marked preoperatively to facilitate the search for the transplanted pancreas in the adherent abdominal structure.
Laparotomy and endoscopic ultrasound-guided pancreas graft biopsy
Biopsies can also be performed via a laparotomy. In 2009, a report was published from Kyushu University, Japan, on 13 cases of biopsy for whom this method was used (13). The University of Oslo, Norway, reported endoscopic ultrasound-guided pancreas graft biopsy using donor-recipient duodenal-duodenal anastomosis (14). Neither of these 2 techniques has been adopted in our center due to greater trauma and different surgical style.
Selection of biopsy strategy
We believe that the selection of biopsy strategy is based on 2 key factors: safety, which is particularly important, and the success rate of sampling. A biopsy can result in serious complications, such as pancreatic leakage, intestinal leakage, and abdominal hematoma, and removal of the transplanted pancreas is unacceptable to both doctors and patients. Laparoscopic biopsy is slightly more invasive but less risky, whereas percutaneous CT combined with ultrasound-guided puncture biopsy is less invasive but more risky. The selection of the biopsy method should be based on the comprehensive evaluation of CT images before the biopsy.
Despite the overall risk of pancreatic biopsy being greater than that of kidney or liver biopsy, its significance is obvious, and treatment must be based on accurate diagnosis. In addition, some morphological changes must have histological evidence, and, as was the case with patient 4 in the present series, without histological support, it is extremely difficult to obtain correct results based on clinical judgment alone.
Although there were only 4 patients in this case study, we explored the options for transplanted pancreas biopsy for homolateral SPK transplantation. Pancreas graft biopsy is currently being performed in a few centers. How to determine the best biopsy strategy requires further investigation, and we hope our preliminary study can contribute to this work.
Conclusions
Many biopsy methods can be used for pancreas graft after SPK transplantation. Different biopsy strategies should be formulated according to the anatomical location of the transplanted pancreas.
Acknowledgments
The first author, Lei Zhang, conducted the study at the Second Affiliated Hospital of Guangzhou Medical University, and now works at the Third Affiliated Hospital of Sun Yat-sen University.
Funding: This work was supported by Key Clinical Specialty of Guangzhou Medical University (No. F03031) and the Guangzhou Key Discipline of Urology.
Footnote
Reporting Checklist: The authors have completed the MDAR and AME Case Series reporting checklists. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-414/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-414/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-414/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-414/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Institutional Review Board of the Second Affiliated Hospital of Guangzhou Medical University (number 2020-hs-43) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015;20:94-102. [Crossref] [PubMed]
- Wan J, Fang J, Li G, et al. Pancreas allograft biopsies procedure in the management of pancreas transplant recipients. Gland Surg 2019;8:794-8. [Crossref] [PubMed]
- Fehrenbach U, Thiel R, Bady PD, et al. CT fluoroscopy-guided pancreas transplant biopsies: a retrospective evaluation of predictors of complications and success rates. Transpl Int 2021;34:855-64. [Crossref] [PubMed]
- Drachenberg CB, Torrealba JR, Nankivell BJ, et al. Guidelines for the diagnosis of antibody-mediated rejection in pancreas allografts-updated Banff grading schema. Am J Transplant 2011;11:1792-802. [Crossref] [PubMed]
- Zhang L, Chen Z, Lai X, et al. The homolateral simultaneous pancreas-kidney transplantation: a single-center experience in China. Ann Transl Med 2019;7:629. [Crossref] [PubMed]
- Steiniger B, Klempnauer J, Wonigeit K. Altered distribution of class I and class II MHC antigens during acute pancreas allograft rejection in the rat. Transplantation 1985;40:234-9. [Crossref] [PubMed]
- Dietze O, Königsrainer A, Habringer C, et al. Histological features of acute pancreatic allograft rejection after pancreaticoduodenal transplantation in the rat. Transpl Int 1991;4:221-6. [Crossref] [PubMed]
- Williams MD, Fei M, Schadde E, et al. Early Experience Using Donor-derived Cell-free DNA for Surveillance of Rejection Following Simultaneous Pancreas and Kidney Transplantation. Transplant Direct 2022;8:e1321. [Crossref] [PubMed]
- Büttner-Herold M, Amann K, Pfister F, et al. Pancreas transplantation-clinic, technique, and histological assessment. Pathologe 2021;42:509-23. [Crossref] [PubMed]
- Malek SK, Potdar S, Martin JA, et al. Percutaneous ultrasound-guided pancreas allograft biopsy: a single-center experience. Transplant Proc 2005;37:4436-7. [Crossref] [PubMed]
- Becker LE, Hallscheidt P, Schaefer SM, et al. A Single-center Experience on the Value of Pancreas Graft Biopsies and HLA Antibody Monitoring After Simultaneous Pancreas-Kidney Transplantation. Transplant Proc 2015;47:2504-12. [Crossref] [PubMed]
- Uva PD, Odorico JS, Giunippero A, et al. Laparoscopic Biopsies in Pancreas Transplantation. Am J Transplant 2017;17:2173-7. [Crossref] [PubMed]
- Kitada H, Sugitani A, Okabe Y, et al. Availability of pancreatic allograft biopsies via a laparotomy. Transplant Proc 2009;41:4274-6. [Crossref] [PubMed]
- Nordheim E, Horneland R, Aandahl EM, et al. Pancreas transplant rejection episodes are not revealed by biopsies of the donor duodenum in a prospective study with paired biopsies. Am J Transplant 2018;18:1256-61. [Crossref] [PubMed]
(English Language Editors: N. Korszniak and J. Gray)


