
Papillary thyroid carcinoma with rare lymph node metastasis between the non-ipsilateral sternocleidomastoid and striate muscles: two case reports and literature review
Highlight box
Key findings
• This case report presents two cases of PTC with contralateral LNSS lymph node metastasis.
What is known and what is new?
• LNSS metastases are mostly ipsilateral to the tumor and has a low metastatic rate.
• We found that one patient had contralateral level LNSS metastasis and the other with bilateral level LNSS metastasis, both of which are rare clinical cases.
What is the implication, and what should change now?
• In clinical practice, surgeons should focus on the evaluation and clearance of LNSS, especially in patients with cancer foci located in the lower pole, cancer foci invading the anterior cervical band muscle, extensive metastasis in the lateral cervical lymph nodes or stages T3/4 and to reduce postoperative recurrence.
Introduction
The incidence of thyroid cancer has increased in recent years. In the cancer statistics report released by the American Cancer Society in 2022, thyroid cancer ranks seventh in the number of new cancer cases in women (1). Differentiated thyroid cancer (DTC) has a better prognosis among thyroid cancers, with a 10-year survival rate of 90%. However, 40–60% of DTC patients have lymph node metastases at the time of diagnosis (2). The current domestic and foreign guidelines recommend lateral neck dissection (LND) (3-5) for patients with suspicious lymph nodes found in the lateral neck. The 2015 ATA guidelines recommend that the dissection extent includes levels II, III, and IV (4). The recommended extent of LND in the guidelines published in China in 2022 includes levels II, III, IV, and VB (6), and the minimum acceptable extent covers levels IIA, III, and IV, but the sternocleidomastoid is not mentioned. Lymph node metastasis between the sternocleidomastoid and sternohyoid muscle (LNSS) was first reported by Sun et al. in 2013 (7). The anterior boundary of the LNSS is the sternocleidomastoid muscle, the posterior boundary is the sternohyoid muscle, the upper border is the intersection of the sternocleidomastoid muscle and the sternohyoid muscle, and the superior border is the intersection of the sternocleidomastoid muscle and the sternohyoid muscle. The inferior boundaries are the suprasternal fossa and clavicle, the external and internal borders of which are the lateral and internal borders of the sternohyoid muscle, respectively. The LNSS is considered to be part of the suprasternal space. Previous literature has reported that the detection rate of LNSS involvement was 63.5–68.3%, and the transfer rate was 14.4–31.81% (8-10). Currently, knowledge of the LNSS requires improvement, and there are few related reports and studies. A survey in Chengdu, China, found that only 53.64% of surgeons paid attention to this area (9). LNSS metastases are mostly ipsilateral to the tumor, and contralateral metastases are rare; this is why LNSS metastasis is easily ignored, especially in the contralateral neck. Level LNSS metastatic lymph nodes are usually not significantly uncomfortable preoperatively and are not easily detected by preoperative physical examination and ultrasound. Once abnormal enlarged lymph nodes in the level LNSS are found preoperatively, intraoperative dissection of this area is required. This case report presents two cases of papillary thyroid carcinoma (PTC) with contralateral LNSS lymph node metastasis. It is hoped that the report of these two rare cases will arouse attention to the level LNSS, especially the contralateral level LNSS. We present the following article in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-715/rc).
Case presentation
Case 1
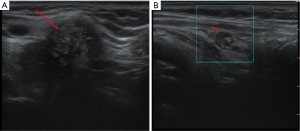
A 63-year-old female patient was admitted to our hospital with a 15-day history of an untreated thyroid nodule. She had no family history of thyroid carcinoma. On physical examination, a mass measuring approximately 3.0 cm × 3.0 cm was palpable on the right side of the anterior midline of the neck. It had poorly defined borders and a hard consistency and moved up and down with swallowing. An ultrasound showed a hypoechoic area near the isthmus of the right lobe, measuring about 2.33 cm × 2.33 cm × 3.13 cm, with an unclear boundary, multiple strong echoes, and no significant blood flow signal (Figure 1A). A hypoechoic lymph node measuring approximately 0.94 cm × 0.55 cm was observed below the lower pole of the right lobe, which was round in shape and less clear in structure. A lymph node was observed in the left cervical LNSS region, measuring about 0.6 cm, with a full shape and unclear structure, and no significant blood flow signal was observed (Figure 1B). Following a fine-needle aspiration (FNA) biopsy of the nodules in the right lobe of the thyroid, the patient was diagnosed with PTC.
During surgery, the nodule near the isthmus of the right lobe was seen to adhere to the anterior cervical muscles. The right lobe of the thyroid gland was removed, together with the lymphatic adipose tissue under the bilateral thyroid, in the tracheoesophageal sulcus, and before the tracheal, and left cervical LNSS. Intraoperative neuromonitoring (IONM) was used throughout the exploration to protect the recurrent laryngeal nerve (RLN) and the external branch of the superior laryngeal nerve (EBSLN) (11,12). The rapid pathological examination showed PTC in the right lobe (3 cm × 2 cm × 1.8 cm) and central and left cervical LNSS lymph node metastases. The patient underwent total thyroidectomy, with significant tumor invasion and central lymph node metastasis. Paraffin pathology revealed PTC in the right lobe (3 cm × 2 cm × 1.8 cm) invading the striated muscle tissue with a 1.2 cm lymph node metastasis at the margin; a nodular goiter in the left lobe; two lymph node metastases in the central area; and two lymph node metastases in the left cervical LNSS (maximal metastases were 0.6 cm) (Figure 2). No postoperative complications were observed, such as hypoparathyroidism, temporary or permanent hoarseness, or reduced tone.
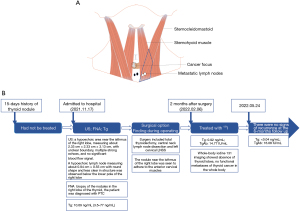
The patient had a preoperative thyroglobulin (Tg) level of 10.09 ng/mL (normal range: 3.5–77 ng/mL), which decreased to 0.92 ng/mL, and thyroglobulin antibodies (TgAb) level of 14.77 IU/mL 2 months after surgery. She was treated with radionuclide therapy and oral 100 mCi iodine. Whole-body iodine 131 imaging showed an absence of thyroid lobes and no functional metastases of thyroid cancer in the whole body, consistent with the postoperative imaging changes. The 18F luorine-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) findings showed soft tissue swelling with slightly increased glucose metabolism in the surgical area and nonspecific inflammatory lymph nodes in the bilateral neck, considered to be caused by postoperative changes. After 6 months of follow-up, there was no recurrence. Tg was <0.04 ng/mL, anti-Tg was 16.08 IU/mL, and thyroid-stimulating hormone (TSH) was 0.115 ng/mL. No adverse or unexpected events were observed during treatment. Her prognosis was good, and there were no signs of recurrence at her 6-month follow-up appointment. A timeline is shown in Figure 2.
Case 2
A 24-year-old female was admitted to our hospital for a physical examination of an anterior cervical mass that had been present for 1 year and not treated. She had no family history of thyroid carcinoma. A mass was palpable in the anterior midline of the neck, measuring approximately 2 cm × 1 cm, with unclear borders and a hard texture. It was situated above the cricoid cartilage plate and moved up and down with swallowing. A color Doppler ultrasound showed hypoechogenicity in the conical lobe of the thyroid, with an unclear boundary of about 1.18 cm × 0.68 cm; the deep layer was closely related to the muscular layer; the blood flow signal was slightly rich; several hypoechoic lymph nodes were observed on the left side of the suprasternal fossa, with the larger one measuring approximately 1.02 cm × 0.5 cm; the shape was full, and the structure was unclear; multiple enlarged lymph nodes were observed between the bilateral sternocleidomastoid muscle and the zoster muscle, and the internal structure of some lymph nodes was unclear. Following an FNA biopsy of the nodules in the conical thyroid lobe, the patient was diagnosed with PTC.
During surgery, a hard mass measuring 1.0 cm × 1.0 cm was palpable at the conical lobe of the thyroid gland with unclear borders, and multiple enlarged lymph nodes were linearly distributed along the linea alba of the neck on the glandular surface of the thyroid isthmus below it (Figure 3). Considering the possibility of malignancy of the thyroid conical lobe mass, a series of lymph nodes between the conical lobe and the inferior part was removed, and the lymph nodes between the bilateral sternocleidomastoid and striate muscles were removed. IONM was used throughout the exploration to protect the RLN and EBSLN (11,12). The rapid pathological results showed three papillary carcinoma nodules in the conical lobe mass, and a lymph node were seen around one of the cancer nodules, which may be metastatic lymph nodes; the lymph nodes between the bilateral sternocleidomastoid muscle and striate muscle were thyroid carcinoma metastases. Considering that the patient needed radionuclide therapy after surgery, a total thyroidectomy was performed.
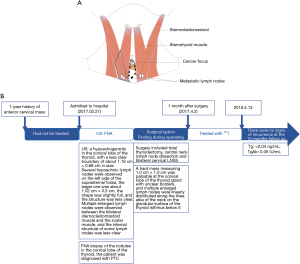
Paraffin pathology revealed local adenomatous hyperplasia of a nodular goiter in the right lobe of the thyroid gland with metastasis of one lymph node at the margin, a nodular goiter in the left lobe of the thyroid gland, local adenomatous hyperplasia, metastasis of three lymph nodes in the central area, papillary carcinoma in a few thyroid tissues in the conical lobe tissue (microscopic diameter 0.3 cm) and one cancerous nodule, focal invasion of the striated muscle, and metastasis of three lymph nodes in the series of lymph nodes below the conical lobe with a maximum diameter of 0.8 cm. A3 immunohistochemical staining revealed CKl9 (+); Galectin-3 (+); CD56 (−); TTF-1 (+); Tg (−), metastasis of three lymph nodes in the left cervical LNSS, and metastasis of four lymph nodes in the right cervical LNSS. One month after surgery, the patient underwent radionuclide therapy and TSH suppression therapy. After 12 months of follow-up, there was no recurrence, and reexamination with Tg and TgAb tests showed 0.04 ng/mL and 0.05 IU/mL, respectively. No adverse or unexpected events were observed during treatment. The patient’s prognosis was good, and there were no signs of recurrence at the 12-month follow-up appointment. A timeline is shown in Figure 3.
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Currently, the deep cervical lymph node subdivision is based on the standard subdivision system for deep cervical lymph nodes designated by the American Head and Neck Association and the American Joint Committee on Cancer (AJCC) (13). The LNSS is located between the superficial and deep cervical lymph nodes but has not been included in the consensus lymph node subregion due to the obstruction of muscles independent of levels IV and VI. LNSS involvement is less frequent, and metastatic lymph nodes are smaller, with the number of LNSS previously reported as 0–7. A study by Zhang et al. found that the mean longest diameter of LNSS metastatic lymph nodes was (0.5±0.12) cm (10). Because LNSS has a concealed location, small metastatic lymph nodes, few cystic degenerations and calcification, and is located in areas with more fat, it is difficult to detect by physical examination and ultrasonography during preoperative evaluation. Conventional computed tomography (CT) will also be affected by the anterior jugular vein enhancement shadows. Moreover, selective cervical lymphadenectomy or modified cervical lymphadenectomy are currently the surgeries of choice, and these procedures can easily overlook the LNSS area due to preservation of the sternocleidomastoid muscle, causing the area to be poorly exposed.
Factors associated with LNSS metastasis:
In recent years, it has been found that LNSS metastasis is mainly associated with the following factors: (I) the cancer lesion is located in the lower pole. In 2021, Song et al. conducted a prospective study of 234 patients who underwent LND and found that 66.67% (56/84) of LNSS metastatic patients had cancer lesions located in the lower pole of the thyroid gland, and 21.67% (39/180) of 180 LNSS non-metastatic patients had cancer lesions located in the lower pole (9). LNSS metastasis has also been associated with cancer foci located in the lower pole in studies by Robbins et al., Dai et al. and Hao et al. (13-15). However, Zhang et al. found no correlation between the location of cancer foci in the lower pole and LNSS metastasis (10), so these findings still need to be confirmed by further studies. (II) Cancer invades the anterior cervical strap muscle. Zhang et al. found that 88.9% (16/18) of patients with LNSS metastasis had cancer foci invading the anterior cervical strap muscle (10). Both patients in our report also had cancer lesions invading the anterior cervical strap muscle. (III) Cervical lymph node metastasis. Sun et al. analyzed 26 patients with LNSS metastasis among 115 patients with cN1 in 2013 and found that lymph node metastasis in levels III (24/26) and IV (24/26) was an independent risk factor for LNSS metastasis (7). In 2017, 54 PTC patients who underwent lymph node dissection in the LNSS region were analyzed again, and it was found that having >4 metastatic lymph nodes in level IV was associated with LNSS metastasis. Hao et al. found that >6 cervical metastatic lymph nodes was an independent risk factor for LNSS metastasis, with a sensitivity of 92.3% and a specificity of 66.7%, and all patients with LNSS metastasis had level VI lymph node metastasis and BRAF mutations (15). Zhang et al. found an increased rate of LNSS metastasis in patients with both central and lateral cervical lymph node metastasis (10). (IV) Large tumor. Sun et al. demonstrated that cancer foci >2.2 cm were associated with LNSS metastasis (8). In Dai et al.’s study, patients with cancer lesions >1 cm were more likely to develop LNSS metastasis (14). (V) Age >45 years; Song et al. conducted a prospective study of 234 patients who underwent LND and found that age >45 years was an independent risk factor for LNSS metastasis (9). At present, there is a lack of large-sample, multicenter studies on the factors associated with LNSS metastasis, and more evidence-based research is needed to confirm these findings. Bilateral LNSS metastases were also found in some patients in the aforementioned study by Song et al. (9) and in five patients in the study by Dai et al. (14), but no further studies have been conducted on this. We report for the first time LNSS metastasis contralateral to cancer foci and bilateral LNSS metastasis. In this report, our Case 1 patient was >45 years old, had cancer lesions >2.2 cm, and lesions invading the anterior cervical ribbon muscle. Although our Case 2 patient was <45 years old and had small cancer foci, cancer foci invaded the anterior cervical strap muscle, and there were more than six cervical lymph node metastases.
LNSS lymph node metastasis pathway
At present, the mechanism of thyroid and anterior cervical lymphatic drainage is not clear. It is generally accepted that lymph node metastasis of PTC occurs stepwise, first to the central group lymph nodes and then to the lateral cervical lymph nodes (13). PTC generally drains to the deep cervical lymph nodes and rarely metastasizes to the superficial cervical lymph nodes, and LNSS is not a common drainage area for thyroid cancer. Currently, there are several main views on the mechanism of LNSS metastasis: (I) LNSS is an important pathway draining the anterior superficial part of the neck to the deep neck. Because cervical lymphatic drainage is mostly distributed along the anterior jugular vein, lymphatic drainage in the superior and inferior hyoid regions of the superficial anterior cervical region is collected from the anterior jugular vein and eventually drains through the LNSS into the jugular angle (10). We suspect that these two LNSS metastases may have been tumor invasions into the anterior cervical lymphatic drainage system via invasion of the anterior cervical strap muscle and hence drained to the LNSS via the anterior cervical lymphatic drainage system around the anterior cervical vein on the superficial surface of the anterior cervical strap muscle. Contralateral LNSS metastasis may occur via the communicating branch of the anterior jugular vein; (II) advanced thyroid cancer metastasizes retrogradely to the superficial cervical lymph nodes due to blockage of the deep cervical lymphatic vessels by the cancer cells; (III) Delphian lymph nodes communicate with the superficial anterior cervical lymph nodes to metastasize to the LNSS (16). Case 2 was a conical lobe carcinoma predisposed to Delphian lymph node metastasis, which may also be one of the paths that caused metastasis to the LNSS in Case 2. (IV) Increased tumor burden in the lateral cervical lymph node metastases, especially in level IV, via fibroadipose tissue migration into the sternocleidomastoid and sternohyoid muscle (17). Unfortunately, neither of these patients had lymphotracers, such as carbon nanoparticle suspension, that were used intraoperatively to visualize the possible lymphatic drainage pathways at the LNSS level. Sun et al. conjectured that LNSS is not congenitally present and may be associated with specific pathological patient features, such as multifocality on the affected side (7). In their 2017 study, they found that patients with LNSS lymph node dissection had lower preoperative serum TSH (2.03 vs. 2.30 mU/L), higher thyroid peroxidase antibody (TPO-Ab) levels (1.92 vs. 1.69 U/mL), and a higher proportion of unilateral multifocality compared with those with LNSS regional dissections of only fibroadipose tissue. Homma et al. speculated that fibroadipose tissue and metastatic lymph nodes in levels III and IV gradually moved in the direction of LNSS due to the daily movements of the neck (18). LNSS was first identified in patients undergoing reoperation, and 12.9% (4/31) of 31 patients with PTC undergoing reoperation were found to have LNSS metastasis in a study by Lv et al. (19). However, Sun et al. compared patients undergoing primary surgery and reoperation and found that there was no statistical significance between the two, thus excluding implantation translocation (7).
Selection of LNSS sweeping
Surgical debulking is the primary treatment for DTC patients with metastatic lymph nodes. The standard and thoroughness of surgical debulking are the main foci of research. There is a clear understanding of debulking of the central and lateral cervical lymph nodes. For DTC CN1a and CN1b, therapeutic lymph node dissection of the central (levels VI–VII) and lateral cervical lymph nodes is recommended. For CN0, European and American guidelines recommend prophylactic central group lymph node dissection (pCND) only for patients with T3, T4, and cN1b (4,20). The pCND procedure is mainly performed in countries in the East Asian region (21). For cN0 patients, the guidelines issued by Japan and China recommend that central compartment dissection may be considered if there are high-risk factors (e.g., T3−T4 lesions, multifocality, family history, or childhood ionizing radiation exposure). Individualized treatment is feasible for low-risk CN0 patients (without high-risk factors) and is chosen according to prognosis and patient wishes (6,22). Our Case 1 thyroid tumor invading the anterior cervical band muscle was T3b stage. Case 2 was also T3b stage, and preoperative examination revealed multiple enlarged lymph nodes in the central compartment identified as cN1a. Both patients underwent central group lymph node dissection (CND). Postoperative paraffin pathology confirmed that both patients had central group lymph node metastases.
LNSS has a low metastatic rate and is not included in the routine surgical anatomical region of the thyroid. However, in clinical practice, surgeons should focus on the evaluation and clearance of LNSS to reduce postoperative recurrence in cases where there is lymph node enlargement in the area of LNSS detected by routine preoperative examination, cancer foci located in the lower pole, cancer foci invading the anterior cervical band muscle, extensive metastasis in the lateral cervical lymph nodes (especially lymph node metastasis in level IV), or stages T3/4 (23). There are no studies on factors associated with metastatic lymph nodes in the contralateral level LNSS. Perhaps we should pay attention to preoperative evaluation of bilateral level LNSS lymph nodes in patients with high risk factors for level LNSS metastasis. Both patients in our study were found to have enlarged lymph nodes in the LNSS area on clinical examination, so LNSS lymph node dissection was performed in both cases, and no bleeding or lymphatic leakage occurred after surgery.
Conclusions
LNSS is difficult to detect during the preoperative examination, but intraoperative exploration and dissection are not difficult. There are no important nerves and blood vessels in the LNSS area, and dissection of this area does not increase the difficulty of surgery and complications. However, attention should be paid to hemostasis and ligation of the proximal end of the vein to avoid the occurrence of postoperative hematoma or anterior cervical lymphorrhea caused by postoperative intermuscular vessels, deep oozing of the sternocleidomastoid muscle, and intermuscular lymphatic vessel injury.
Also, for patients with unilateral cancerous lesions in the thyroid, emphasis should be placed not only on the assessment of the affected cervical lymph nodes but also on the contralateral cervical lymph nodes. Patients with metastatic LNSS without lateral cervical lymph node dissection should be closely monitored during the follow-up period for early detection and management.
Acknowledgments
Funding: This work was supported by the Wu Jieping Medical Foundation (No. 320.6750.2020-06-26) and funded by Science and Technology Research Project of Education Department of Jilin Province, China (Nos. JJKH20190066KJ, JJKH20221065KJ).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-715/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-715/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Zhou B, Qin J. High-risk factors for lymph node metastasis in contralateral central compartment in unilateral papillary thyroid carcinoma (cT1N0). Eur J Surg Oncol 2021;47:882-7. [Crossref] [PubMed]
- Xu ZG, Liu SY. Expert consensus on lymph node dissection in the lateral cervical region for differentiated thyroid cancer (2017 version). Chinese Journal of Practical Surgery 2017;37:985-91.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81:1-122. [Crossref] [PubMed]
- Health Commission Of The People's Republic Of China N. National guidelines for diagnosis and treatment of thyroid cancer 2022 in China (English version). Chin J Cancer Res 2022;34:131-50. [Crossref] [PubMed]
- Sun G, Wang Y, Zhu Y, et al. Lymph node metastasis between sternocleidomastoid and sternohyoid muscle in clinically node-positive papillary thyroid carcinoma. Head Neck 2013;35:1168-70. [Crossref] [PubMed]
- Sun GH, Qu N, Hu JQ, et al. Risk for metastasis of lymph node between sternocleidomastoid and sternohyoid muscle in papillary thyroid cancer. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017;52:253-8. [Crossref] [PubMed]
- Song L, Zhou J, Chen W, et al. Lymph node metastasis between the sternocleidomastoid and sternohyoid muscle in papillary thyroid carcinoma patients: A prospective study at multiple centers. Asian J Surg 2021;44:1043-9. [Crossref] [PubMed]
- Zhang HL, Jie Chen, Huang WX, et al. Analysis of clinical characteristics of lymph node metastasis between papillary thyroid cancer Sternocleidomastoid muscle and sternohyoid muscle. Chinese Journal of Otorhinolaryngology and Skull Base Surgery 2014;20:301-4.
- Ling Y, Zhao J, Zhao Y, et al. Role of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid and parathyroid surgery. J Int Med Res 2020;48:300060520952646. [Crossref] [PubMed]
- Potenza AS, Araujo Filho VJF, Cernea CR. Injury of the external branch of the superior laryngeal nerve in thyroid surgery. Gland Surg 2017;6:552-62. [Crossref] [PubMed]
- Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg 2002;128:751-8. [Crossref] [PubMed]
- Dai XS, Guo L, Zhao JZ, et al. Analysis of neck lymph node dissection in 68 cases of thyroid papillary carcinoma. J Oncol 2020;26:133-8.
- Hao SL, Sun HQ, Liu XC, et al. Clinical significance of lymphatic metastasis between sternocleidomastoid and sternohyoid muscle in papillary thyroid cancer. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017;52:755-9. [Crossref] [PubMed]
- Lee YC, Shin SY, Kwon KH, et al. Incidence and clinical characteristics of prelaryngeal lymph node metastasis in papillary thyroid cancer. Eur Arch Otorhinolaryngol 2013;270:2547-50. [Crossref] [PubMed]
- Ji QH, Sun GH, Wang Y, et al. Pay attention to the management of Sternocleidomastoid muscle sternohyoid muscle lymph nodes in papillary thyroid carcinoma. Chinese Journal of Cancer 2018;28:487-90.
- Homma A, Hatakeyama H, Mizumachi T, et al. Lymph node metastasis in the suprasternal space from thyroid papillary cancer. Int Canc Conf J 2015;4:57-60.
- LV CZ. Analysis of reoperation after cervical lymph node dissection for thyroid papillary carcinoma. Chinese Journal of Practical Surgery 2019;39:722-4.
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1856-83. [Crossref] [PubMed]
- Guidelines Working Committee of Chinese Society of Clinical Oncology. Guidelines of Chinese Society of Clinical Oncology (CSCO) Differentiated Thyroid Cancer. J Cancer Control Treat 2021;34:1164-201.
- Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: Core questions and recommendations for treatments of thyroid cancer. Endocr J 2020;67:669-717. [Crossref] [PubMed]
- Zhai Y, Ruan L. The Necessity of Lymph Node Dissection Between Sternocleidomastoid and Sternohyoid Muscles in pN1b Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne) 2022;13:865621. [Crossref] [PubMed]


