
Two cases report of secretory carcinoma of the salivary gland in the lung: one primary and one metastatic after many years
Highlight box
Key findings
• We found two cases of secretory carcinoma of the lung.
What is known and what is new?
• Secretory carcinoma is known to be primary in breast, parotid gland and other parts.
• It is extremely rare to occur in lung, especially primary in lung.
What is the implication, and what should change now?
• In the bronchial area, we should think of the possibility of secretory carcinoma when we see low-grade cribriform carcinoma. Breast-like secretory carcinoma can also be primary in the lung.
Introduction
The Fourth World Health Organization (WHO) Classification of Head and Neck Tumors published in 2017 officially recognized secretory carcinoma of the salivary gland (SCSG) as an independent disease. SCSG is described as a generally low-grade salivary gland carcinoma characterized by its morphological resemblance to mammary secretory carcinoma and ETV-6-NTRK3 gene fusion (1). Salivary gland-type tumors of the lung are uncommon, accounting for <1% of all primary lung neoplasms (2). In recent years, the spectrum of pulmonary salivary gland-type tumors has been expanding (3). However, to the best of our knowledge, primary lung SCSG has rarely been reported. Herein, we report two cases of SCSG in the lung; one case was a primary tumor and the other case involved a suspected metastatic tumor. We present the following article in accordance with the CARE checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-709/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
The patient was a 56-year-old male who was admitted to the Respiratory Department at Shanxi Tumor Hospital (Taiyuan, Shanxi Province, China) for a left upper lobe mass on March 29, 2022. He presented with obstructive atelectasis, accompanied by fever, cough, and yellow thick sputum. There was no hemoptysis, chest constriction, shortness of breath, or chest or back pain. The patient had a poor appetite and sleep, normal urine and feces, and a weight loss of approximately 3 kg. He had a negative history of surgery, hypertension, diabetes, and heart disease, and no history of hepatitis, tuberculosis, and other infectious diseases [hepatitis B virus (HBV) (−), hepatitis C virus (HCV) (−), HIV (−), (Epstein-Barr virus) EBV (−)]. Moreover, he denied a history of blood transfusion as well as food and drug allergies and had no familial hereditary, immune, or mental diseases.
Chest computed tomography (CT) showed obstructive atelectasis in the left upper lobe of the lung near the hilum; multiple lymph nodes of different sizes were observed in the mediastinum and left hilum, with the largest measuring 0.8 cm in diameter. Color Doppler ultrasound of the neck suggested several lymph nodes in the IV area of the left neck measuring 0.55×0.44–1.17×0.86 cm. Single-photon emission computed tomography (SPECT) whole-body bone scanning revealed point-form increased bone metabolism in the left anterior eighth costal bone.
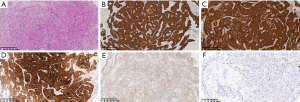
After obtaining the patient’s informed consent, the surgeon performed a fiberoptic bronchoscopy, which revealed a new growth in the upper bronchus of the right lung. Two tissue samples were obtained by biopsy. Patchy tumor tissue was detected under the bronchial mucosa containing round and polygonal tumor cells, unclear boundaries, abundant eosinophilic cytoplasm, uniform nuclei, and little atypia. The tumor cells were arranged in nests, and glandular lumen and eosinophilic secretions were observed in the lumen (Figure 1A). Immunohistochemistry was positive for AE1/AE3 (Figure 1B), Keratin7 (CK7), S-100 (Figure 1C), mammaglobin (Figure 1D), and pan-TRK (Figure 1E), and negative for thyroid transcription factor-1 (TTF-1), napsin-A, synaptophysin (SYN), chromogranin A (CGA), and discovered on GIST-1 (Dog-1). The MKI-67 (Ki-67) proliferation index was 2% (Figure 1F). Although the morphology and immunomarker results were consistent with SCSG, fluorescence in situ hybridization (FISH) was negative for ETV-6 rearrangement.
After a cycle of pemetrexed + carboplatin chemotherapy, the lung tumor shrank markedly. The patient plans to have surgery after further reduction in the size of the tumor.
This rare primary case is unique in that both histological morphology and immune markers support the diagnosis of SCSG, but lacks the typical ETV-6 gene rearrangement. We speculate that this case may carry a rare genetic mutation.
Case 2
A 50-year-old man presented to a local hospital with left parotid gland enlargement and was admitted in June 2021. A mumps removal operation was performed at the local hospital. CT examination revealed a mass in the anterior upper lobe of the left lung. He was admitted to the Department of Thoracic Surgery of Shanxi Tumor Hospital (Taiyuan, Shanxi Province, China) on June 25, 2021, for treatment of the lung mass. The patient reported that in 1985 when he was 14 years old, he underwent left parotid pleomorphic adenoma resection at the local hospital. In 2005, when he was 34 years old, he underwent resection of a left parotid malignant pleomorphic adenoma in our hospital. After the surgery, the patient received radiation therapy and suffered discharge from an abscess in the left ear. The timeline figure of his disease is shown in Figure 2.
Physical examination showed postoperative changes of the mass in the left parotid gland area, no enlargement of superficial lymph nodes in the body, clear breath sounds in both lungs, no dry or wet rales, and a normal heart rate of 73 beats/min. No pathological murmurs were heard in the auscultation area of heart valves. The abdomen was flat and soft, without tenderness or rebound pain, and the liver and spleen were not palpable under the ribs.
A quasi-circular mass with irregular edges measuring 2.07 cm × 2.05 cm and a low-density liquefaction necrotic area were detected in the upper lobe of the left lung. These findings were consistent with peripheral lung cancer in the upper lobe of the left lung. Magnetic resonance imaging (MRI) examination showed an absent left parotid gland, left maxillofacial soft tissue swelling, and edema of the muscle, which was considered to be a possible infection. In addition, bilateral ethmoid sinus and maxillary sinusitis, a right maxillary sinus cyst, and left middle ear mastoiditis were also observed.
To confirm the diagnosis, a CT-guided puncture of the left lung mass was performed after obtaining informed consent. The biopsy results led to a diagnosis of salivary adenocarcinoma by pathological examination. The patient then underwent a thoracoscopic wedge resection of the upper lobe of the left lung and closed thoracic drainage. Intraoperatively, a mass measuring 3 cm × 2.5 cm × 2.5 cm was found in the posterior segment of the upper lobe apex of the left lung.
During postoperative analysis of the surgical specimens, a visible mass under the pleura at a distance of 2 cm was visualized. The mass was grey and sallow, and the surrounding tissue boundaries were not clear. Under the microscope, a well-defined mass was detected in the lung tissue (Figure 3A). The tumor cells were round and oval, with a moderately atypical nucleus and rich eosinophilic and basophilic cytoplasm. The tumor cells were arranged in a glandular, sieve, and papillary shape and basophilic secretions were observed in the glandular cavity (Figure 3B).
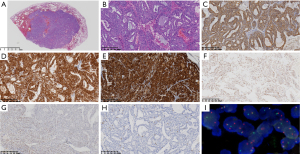
Immunohistochemistry was positive for AE1/AE3 (Figure3C), CK7, S-100 (Figure 3D), and mammaglobin (Figure 3E); partially positive for ER (Figure 3F) and pan-TRK (Figure 3G); and negative for TTF-1, napsin-A, SYN, CGA, P63, P40, and Dog-1. The Ki-67 proliferation index was approximately 3% (Figure 3H). The morphology and immunolabeling results suggested that the tumor was SCSG. FISH examination showed ectopic ETV-6 gene rearrangement (Figure 3I). The patient was finally diagnosed with SCSG and did not receive radiotherapy and chemotherapy after surgery. A follow-up examination at 11 months showed that the parotid gland had healed; there was no recurrence in the lung and the patient was in good condition.
This case is unique in that it has a complex and lengthy history of parotid gland tumors. Thirty-seven years later, the parotid tumor recurred and metastasized to the lung, presenting as a peripheral mass. Despite repeated recurrences and metastases, it is still a low-grade form. In terms of histomorphology, immunohistochemical expression and ETV-6 rearrangement showed typical SCSG.
Discussion
History
SCSG is a recently discovered salivary gland tumor and was first reported by Skálová (4) in 2010. Since its morphological, immunophenotypical, and molecular characteristics are similar to those of breast secretory carcinoma, it was named salivary gland secretory carcinoma (MGSC) at that time (5,6). As a member of the new classification of salivary gland malignant tumors, it was officially listed in the WHO Head and Neck Tumor Classification (4th edition) and referred to as secretory carcinoma. SCSGs of other organs have also been reported, such as the minor salivary glands around the sinuses (7), lips (8), and buccal mucosa (9), as well as the thyroid gland (10) and lungs. It often presents as a cystic-solid mass and the boundary is not clear. The structural morphology is diverse, including microcapsule, papillary capsule, solid, tubular, large capsule, and follicular types (11). The same tumor can contain numerous different histological types, which have been diagnosed as acinar cell carcinoma in the past.
Clinicopathological and pathological features
SCSG usually presents in adults, with a mean patient age of 46.5 years (range, 10–86 years), and no differences between the sexes have been observed (12). The mammaglobin, S-100, and pan-TRK proteins are often expressed in tumor cells, and the ETV-6-NTRK3 fusion gene is frequently detected. However, in clinical practice, pathologies that support the diagnosis of SCSG are frequently detected by morphological analysis as well as immunohistochemistry with no ETV-6 gene rearrangement identified by molecular analysis. Several studies have reported that in addition to ETV-6-NTRK rearrangement, other rearrangement patterns were found in a few cases, including ETV-6-RET and ETV-6-MET (13), VIM-RET (14), EGFR-SEPT14 (15), CTNNA1-ALK (16), as well as dual ETV-6-NTRK3 and ETV-6-MAML3 (17). The presence of these rare gene mutations in SCSG cannot be detected using a FISH ETV-6 breakage probe alone. In this study, classical ETV-6 gene rearrangement was detected in Case 2. However, in Case 1, although the morphology and immune markers supported the diagnosis of SCSG, ETV-6 gene rearrangement was not detected by FISH. We speculate that this case may involve a rare gene variation.
The diagnosis of SCSG needs to make a comprehensive judgment by combining the tissue morphology, the results of immune markers, molecular detection, clinical manifestations and imaging findings. If the results are consistent in other aspects, the possibility of rare gene variation should be considered if the results of molecular detection are inconsistent.
Identification of the origin or metastasis
The clinical treatment of primary SCSG differs from that of metastatic SCSG; therefore, differential diagnosis of the two conditions is critical. Primary or metastatic SCSG often poses diagnostic challenges, especially in small biopsy and cytology samples. The clinical characteristics, histomorphology, immunophenotypic features, and molecular alterations are crucial for the diagnosis and differential diagnosis of pulmonary SCSG (18).
In terms of clinical features, it is necessary to carefully screen for the existence of in situ lesions in other organs of the body (e.g., the parotid gland and minor salivary gland, among others). Since primary secretory carcinomas of the lung can originate from the bronchial mucous gland, the mass is often located in the central bronchus. The tumor in Case 1 was located in the bronchus of the upper lobe of the left lung, and there were no in situ lesions in other organs after systemic examination, suggesting a diagnosis of primary SCSG. Case 2 had a history of malignant pleomorphic adenoma of the parotid gland. Although we were unable to review the 2005 slices and SCSG was not detected at the time, it is likely that the malignant pleomorphic adenoma originally detected was in fact SCSG. In addition, the disease progression of the patient for 14 years was in line with the low-grade malignant and slow progression characteristics of SCSG.
Histological morphology under the microscope can also provide clues for the diagnosis of primary or metastatic SCSG (19). In this study, the tumor in Case 1 was detected under the bronchial mucosa under the microscope, and atypical hyperplastic lesions were present adjacent to the cancer, which supports the diagnosis of primary SCSG. However, the tumor in Case 2 was located far from the bronchus and under the visceral pleura, which supports a diagnosis of metastatic SCSG.
Although immunophenotype and molecular phenotype are comparable between primary and metastatic SCSGs in theory, organ specificity cannot be ruled out and needs to be investigated further.
Differential diagnosis
In clinical practice, it is important to consider the following differential diagnoses.
Acinar cell carcinoma
Similar to SCSG, acinar cell carcinoma presents as a variety of histological forms, including solid, microcystic, papillary cystic, and follicular types. Assisted diagnosis with the aid of immunohistochemistry and molecular detection are frequently needed. Acinar cell carcinoma does not express or only shows focal expression of the S-100 protein, mammaglobin, and pan-TRK, and does not express Dog-1. In this study, both cases expressed S-100, mammaglobin, and pan-TRK, but did not express Dog-1. Also, ETV-6 gene rearrangements are detected in SCSG, but not in acinar cell carcinoma (20).
Ductal carcinoma of the salivary gland
Ductal carcinoma of the salivary gland mainly occurs in large salivary glands, such as the parotid gland, and is characterized by a dilated duct-like structure and parietal secretion, or papillary protuberance of the ductal epithelium, and exhibits lack of a fibrous connective tissue axis. Immunohistochemical staining shows strong expression of the Smur100 protein and mammaglobin, which may lead to its misdiagnosis as secretory carcinoma. However, the cells around the tumor express myoepithelium (21) and there is no ETV-6 gene rearrangement.
Treatment
Secretory carcinoma is a low-grade malignant tumor with rare lymph node metastasis; however distant metastasis and death are possible (22). In this study, there was no distant metastasis in either of the two cases, although both had enlarged lymph nodes and suspected metastasis. Case 1 underwent chemotherapy to prepare for surgery, whereas Case 2 underwent thoracoscopic mass resection without lymph node dissection. This patient (Case 2) did not receive radiotherapy or chemotherapy postoperatively, and during the 11 months of follow-up, there was no metastasis or recurrence. Considering that secretory carcinoma is a low-grade malignancy, surgical excision is the best treatment. Both the existing studies and the two cases we reported showed no need for postoperative chemoradiotherapy.
Conclusions
We found that secretory carcinoma can originate from the small salivary gland tissue under bronchial mucosa, and was different from metastatic secretory carcinoma in terms of history, site of onset, age of onset, and size of mass. Secretory carcinoma occurring in the lung was usually of low grade, and the main treatment was surgical resection. Secretory carcinoma has special microscopic histological morphology, immunomarker expression and molecular characteristics. The molecular characteristics are usually ETV-6-NTRK3 gene fusion, and some rare molecular variations also can be seen by chance.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-709/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-709/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Naggar AK, Chan JKC, Grandis JR, et al. WHO classification of Head and Neck tumours. Revised 4th edn. Lyon, France: IARC; 2017:177-9.
- Wang M, Gilani S, Xu H, et al. Salivary Gland-type Tumors of the Lung. Arch Pathol Lab Med 2021;145:1379-86. [Crossref] [PubMed]
- Huang HC, Zhao L, Cao XH, et al. Primary salivary gland tumors of the lung: Two cases date report and literature review. Respir Med Case Rep 2021;32:101333. [Crossref] [PubMed]
- Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010;34:599-608. [Crossref] [PubMed]
- Sethi R, Kozin E, Remenschneider A, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope 2014;124:188-95. [Crossref] [PubMed]
- Luo W, Lindley SW, Lindley PH, et al. Mammary analog secretory carcinoma of salivary gland with high-grade histology arising in hard palate, report of a case and review of literature. Int J Clin Exp Pathol 2014;7:9008-22.
- Hyrcza MD, Andreasen S, Melchior LC, et al. Primary Secretory Carcinoma of the Lacrimal Gland: Report of a New Entity. Am J Ophthalmol 2018;193:178-83. [Crossref] [PubMed]
- Kratochvil FJ 3rd, Stewart JC, Moore SR. Mammary analog secretory carcinoma of salivary glands: a report of 2 cases in the lips. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:630-5. [Crossref] [PubMed]
- Paudel D, Nishimura M, Adhikari BR, et al. Secretory Carcinoma of Minor Salivary Gland in Buccal Mucosa: A Case Report and Review of the Literature. Case Rep Pathol 2019;2019:2074504. [Crossref] [PubMed]
- Desai MA, Mehrad M, Ely KA, et al. Secretory Carcinoma of the Thyroid Gland: Report of a Highly Aggressive Case Clinically Mimicking Undifferentiated Carcinoma and Review of the Literature. Head Neck Pathol 2019;13:562-72. [Crossref] [PubMed]
- Baneckova M, Agaimy A, Andreasen S, et al. Mammary Analog Secretory Carcinoma of the Nasal Cavity: Characterization of 2 Cases and Their Distinction From Other Low-grade Sinonasal Adenocarcinomas. Am J Surg Pathol 2018;42:735-43. [Crossref] [PubMed]
- Huang T, McHugh JB, Berry GJ, et al. Primary mammary analogue secretory carcinoma of the lung: a case report. Hum Pathol 2018;74:109-13. [Crossref] [PubMed]
- Skálová A, Stenman G, Simpson RHW, et al. The Role of Molecular Testing in the Differential Diagnosis of Salivary Gland Carcinomas. Am J Surg Pathol 2018;42:e11-27. [Crossref] [PubMed]
- Skálová A, Banečkova M, Thompson LDR, et al. Expanding the Molecular Spectrum of Secretory Carcinoma of Salivary Glands With a Novel VIM-RET Fusion. Am J Surg Pathol 2020;44:1295-307. [Crossref] [PubMed]
- Hernandez-Prera JC, Holmes BJ, Valentino A, et al. Macrocystic (Mammary Analogue) Secretory Carcinoma: An Unusual Variant and a Pitfall in the Differential Diagnosis of Cystic Lesions in the Head and Neck. Am J Surg Pathol 2019;43:1483-92. [Crossref] [PubMed]
- Na K, Hernandez-Prera JC, Lim JY, et al. Characterization of novel genetic alterations in salivary gland secretory carcinoma. Mod Pathol 2020;33:541-50. [Crossref] [PubMed]
- Guilmette J, Dias-Santagata D, Nosé V, et al. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol 2019;83:50-8. [Crossref] [PubMed]
- Hoda RS, Brogi E, Pareja F, et al. Secretory carcinoma of the breast: clinicopathologic profile of 14 cases emphasising distant metastatic potential. Histopathology 2019;75:213-24. [Crossref] [PubMed]
- Tang H, Zhong L, Jiang H, et al. Secretory carcinoma of the breast with multiple distant metastases in the brain and unfavorable prognosis: a case report and literature review. Diagn Pathol 2021;16:56. [Crossref] [PubMed]
- Zhou W, Han X, Fang Y, et al. Clinical Analysis of Acinar Cell Carcinoma of the Pancreas: A Single-Center Experience of 45 Consecutive Cases. Cancer Control 2020;27:1073274820969447. [Crossref] [PubMed]
- Mnejja M, Kallel S, Thabet W, et al. Ductal carcinomas of the parotid gland. Cancer Radiother 2021;25:155-60. [Crossref] [PubMed]
- Pang Y, Sun L, Liu H, et al. Differential diagnosis and treatment of salivary secretory carcinoma and acinic cell carcinoma. Oral Oncol 2021;119:105370. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



