
The expression of androgen receptor in triple-negative breast cancer and the effect of a traditional Chinese medicine formula on disease-free survival
Introduction
Breast cancer is the most common cancer diagnosed in women, and is the 2nd leading cause of cancer death after lung cancer (1). Triple-negative breast cancer (TNBC) is highly malignant and is noted for the non-expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). In breast cancer patients, the rate of TNBC is 15–20% (2,3). At present, except for short-term 4–8 cycles chemotherapy, there is no other effective drug to reduce recurrence and metastasis in the long term. The molecular subtyping of TNBC is critical to guiding individualized patient treatment (4).
TNBC is a heterogeneous disease with multiple subtypes (5), including immunomodulatory (IM), mesenchymal stem-like (MSL), basal-like 1 and 2 (BL1 and BL2), mesenchymal (M), and androgen receptor (AR) signaling. In 2016, it was further divided into BL1, BL2, M, and luminal androgen receptor (LAR) subtypes (6). The AR is a defining feature of LAR (7). AR is a steroid receptor belonging to the nuclear receptor superfamily. The AR gene, located on the X chromosome, is encoded by 8 exons and 7 introns for 90-kb long (8-10). It can be observed in many tissues, such as skeletal muscle, testes, prostate, and breast and uterus tissues. AR expression is observed in 25–75% of TNBCs (11).
A large amount of clinical data has confirmed the poor prognosis of TNBC patients, which is the result of high tumor invasion and the absence of targeted inhibitors (12). With no drug-targetable receptors, endocrine and HER2-targeted therapies are not feasible for TNBC (13,14). To date, no targeted treatment for TNBC patients has been approved by the US Food and Drug Administration. After early diagnosis, the standard non-surgical treatment for TNBC patients is chemotherapy or chemotherapy combined with radiotherapy (13). Until recently, the main treatment for TNBC was cytotoxic chemotherapy. However, the effect of chemotherapy on AR-positive TNBC patients is not encouraging.
Knowledge of the subtypes of TNBC will provide better prognosis and more possibilities for personalized treatment (15). Lehmann et al. (16) compared the sensitivity of TNBC cell lines and basal-like cell lines to the AR antagonist bicalutamide and an Hsp90 inhibitor. The results showed that AR is the major driving factor of LAR tumors and has the potential to be a therapeutic target for this TNBC subtype. As with hormone-sensitive prostate cancer, the targeting of ARs has been considered an effective treatment strategy for AR-positive TNBC patients (17).
The use of traditional Chinese medicine (TCM) in breast cancer has a long history. TCM is widely used at our breast disease center. The “Ruyan” formula contains Huangqi (30 g), Dangshen (18 g), Baizhu (9 g), Fuling (12 g), Shashen (15 g), Maidong (15 g), Xianlingpi (15 g), Ezhu (30 g), Shijianchuan (10 g), and Gancao (6 g). This prescription helps to supply Qi and is beneficial to the spleen, coordinates the Chong and conception vessels and shrinks tumors.
In this retrospective research, we examined the expression of AR in TNBC and the effect of the TCM formula on disease-free survival (DFS). We present the following article in accordance with the REMARK reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-508/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of The First Affiliated Hospital of Zhejiang Chinese Medical University (No. 2016-KL-020-02) and informed consent was taken from all the patients.
Patients
Patients who underwent breast surgery at The First Affiliated Hospital of Zhejiang Chinese Medical University from January 2017 to December 2019 were enrolled in this study. The tumor tissue biopsied during the operation was preserved in formalin solution and sent for an immunohistochemical examination at the end of the surgery.
Inclusion and exclusion criteria
Inclusion criteria
To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have primary breast cancer diagnosed as malignant epithelial tumor (breast cancer) by pathological examination after surgical treatment based on the following immunohistochemical (IHC) results: ER <1%, PR <1%, HER-2 (–/1+/2+)/FISH (fluorescence in situ hybridization) without amplification and any AR expression as per the ASCO/CAP (American Society of Clinical Oncology/College of American Pathologists) guidelines for the hormone receptor detection of breast cancer and the HER-2 detection guidelines for breast cancer (2020 edition); (II) be female and aged 18–75 years (including 18 and 75 years old); (III) have no organ metastasis; (IV) have no diagnosis of schizophrenia, depression, or other mental system disease; and (V) agree to voluntarily participated in this clinical study and sign the informed consent form.
Exclusion criteria
Patients were excluded from the study if they met any of the following exclusion criteria: (I) did not meet any of the inclusion criteria; and/or (II) did not undergo surgery at our hospital.
Data
We gathered information about the TNBC patients from our electronic medical record system. The identity information of all patients was kept confidential.
Baseline
The TNBC Patients’ baseline information consisted of age, weight, body mass index (BMI), marital history, fertility circumstance, and family history.
Clinical data
The TNBC patients’ clinical data consisted of surgical option, tumor size, axillary lymph node (ALN) status, and tumor grade.
Pathological data
The TNBC patients’ pathological data consisted of pathological staging, ER, PR, HER-2, FISH, AR, Ki-67, P53, and P63.
Treatment information
The treatment information consisted of chemotherapy, radiotherapy, and TCM.
Survival information
The patients were followed-up by an electronic medical record system query, telephone contact, and other methods to obtain data on their survival status.
Groups
- AR-positive group: the TNBC patients whose IHC results showed AR ≥1%.
- AR-negative group: the TNBC patients whose IHC results showed AR <1%.
- TCM group: after the operation, in addition to a comprehensive treatment with western medicine, the patients in the TCM group took the Ruyan formula orally for >6 months. The dose was adjusted according to the specific situation of each patient .
- Non-TCM group: patients in this group only received western medicine after the operation.
Observation target
Prognostic factors
This study sought to examine whether age, marital history, fertility circumstance, family history, surgical option, tumor size, ALN, tumor grade, chemotherapy, radiotherapy, Ki-67, AR expression, p53, and p63 were risk factors affecting the prognosis of TNBC patients.
DFS
DFS was defined as running from the beginning of the randomization for this study to disease recurrence or metastasis. We compared the DFS of the AR-positive group and the AR-negative group, and examined the effect of AR on TNBC. We also compared the DFS of the TCM group and the non-TCM group and evaluated the effect of the Ruyan formula on recurrence and metastasis in TNBC patients.
The adverse effects of TCM
The adverse effects of TCM were defined as unexpected and harmful reactions that occurred when patients were taking the Ruyan prescription according to the doctor’s advice. The adverse effects included side effects, toxic reactions, dependence, addiction, idiosyncratic reactions, teratogenesis, carcinogenesis, and mutagenesis. The safety of the Ruyan prescription was evaluated by observing the adverse effects of the TCM during the follow-up period.
Statistical analysis
The statistical analysis was carried out in SPSS (version 25.0). The means, standard deviations, medians, and interquartile ranges (IQRs) were calculated for the continuous variables. The counts and percentages were calculated for the dichotomous and polychotomous variables. T-tests and chi-square test were used to compare the baseline demographic and clinical characteristics of the patients. Fisher’s exact test was used when the chi-square test was unavailable. A logistic regression model and Kaplan-Meier survival estimates were used to test the relationship between the prognosis and AR expression levels. All P values <0.05 were considered statistically significant.
Results
Process of inclusion and exclusion
We first identified 823 breast cancer patients, and then collected the clinical data of the 92 patients with TNBC. Based on their pathology reports, we excluded 5 patients (1 who was pathologically diagnosed with a phyllodes tumor, and 4 who did not undergo surgery at our hospital). A flow chart of the inclusion criteria for this study is shown in Figure 1.
Baseline information
There were 87 TNBC pathological reports of AR expression. At present, tumors with nuclear staining of 1% or more are clinically accepted as AR positive. However, this standard has not been widely accepted. We used an AR value ≥1% as the cut-off for the AR index (13). A total of 54 patients were assigned to AR-negative expression group and 33 patients to the AR-positive expression group. The positive rate of AR expression was 37.9%.
The mean age of the AR-negative patients was 49.4 years old [standard deviation (SD) =±13.1]. This was significantly younger than the mean age of patients in the AR-positive group (57.2 years old; SD =±9.3) (see Figure 2). Patients in the AR-positive group (60.7 kg; SD =±5.5) had a higher weight than those in the AR-negative group (56.8 kg; SD =±6.0) (see Figure 3). In relation to the other baselines and demographic characteristics, there was no difference between the two groups. The most used chemotherapy regimen was the EC-T regimen, which contains Epirubicin [100 mg/m2, intravenous drip (ivgtt, IV) day 1], Cyclophosphamide (600 mg/m2, IV day 1), cycled every 21 days for 4 cycles followed by Docetaxel (100 mg/m2, IV on day 1), cycled every 21 days for 4 cycles. A total of 22 patients (22.8%) used this regimen. The basic information of the patients is listed in Table 1.
Table 1
| Characteristics | AR positive (n=33) | AR negative (n=54) | χ2 | P value |
|---|---|---|---|---|
| BMI (kg/m2), n (%) | 0.001 | 1.000 | ||
| ≤18.5 | 0 (0.0) | 0 (0.0) | ||
| 18.5–25 | 30 (90.9) | 49 (90.7) | ||
| ≥25 | 3 (9.1) | 5 (9.3) | ||
| Age group (years), n (%) | 3.851 | 0.064 | ||
| ≤44 | 3 (9.1) | 20 (37.0) | ||
| 45–59 | 18 (54.5) | 22 (40.7) | ||
| ≥60 | 12 (36.4) | 12 (22.3) | ||
| Marital history, n (%) | 1.464 | 0.703 | ||
| Married | 33 (100.0) | 51 (94.4) | ||
| Unmarried | 0 (0.0) | 2 (3.7) | ||
| Widowed | 0 (0.0) | 1 (1.9) | ||
| Fertility circumstance, n (%) | 1.152 | 0.160 | ||
| Porous | 33 (100.0) | 50 (92.4) | ||
| Non-porous | 0 (0.0) | 4 (7.6) | ||
| Family history, n (%) | 0.127 | 1.000 | ||
| Yes | 1 (3.0) | 1 (1.9) | ||
| No | 32 (97.0) | 53 (98.1) | ||
| Surgical option, n (%) | 0.400 | 0.498 | ||
| Mastectomy | 22 (66.7) | 31 (57.4) | ||
| Lumpectomy | 11 (33.3) | 23 (42.6) | ||
| Tumor size, n (%) | 0.859 | 0.693 | ||
| T1 | 4 (12.1) | 5 (9.3) | ||
| T2 | 17 (51.5) | 24 (44.4) | ||
| T3 | 12 (36.4) | 25 (46.3) | ||
| ALN status, n (%) | 1.076 | 0.950 | ||
| N0 | 0 (0.0) | 1 (1.9) | ||
| N1 | 27 (81.8) | 40 (74.1) | ||
| N2 | 4 (12.1) | 9 (16.7) | ||
| N3 | 2 (6.1) | 4 (7.4) | ||
| Tumor grade, n (%) | 0.330 | 0.507 | ||
| I | 0 (0.0) | 0 (0.0) | ||
| II | 13 (39.4) | 26 (48.1) | ||
| III | 20 (60.6) | 28 (51.9) | ||
| Chemotherapy, n (%) | 0.661 | 1.000 | ||
| Yes | 30 (90.1) | 49 (90.7) | ||
| No | 3 (9.9) | 5 (9.3) | ||
| Radiotherapy, n (%) | 2.165 | 0.345 | ||
| Yes | 11 (33.3) | 33 (61.1) | ||
| No | 22 (66.7) | 21 (38.9) | ||
| Relapse and metastasis, n (%) | 2.270 | 0.119 | ||
| Yes | 2 (6.1) | 11 (20.4) | ||
| No | 31 (93.9) | 43 (79.6) | ||
| TCM treatment, n (%) | 0.524 | 0.469 | ||
| Yes | 27 (81.8) | 40 (74.1) | ||
| No | 6 (18.2) | 14 (25.9) |
BMI, body mass index; ALN, axillary lymph node; TCM, traditional Chinese medicine; AR, androgen receptor.
Factors influencing AR expression
To analyze the relationship between patients’ characteristics and AR expression, we used the chi-square test. We found that an AR-positive expression level was correlated with an older age (P=0.006), higher weight (P=0.006), and lower Ki-67 expression (P=0.031). The correlations between the patients’ characteristics and their AR expression levels are set out in Table 2.
Table 2
| Characteristics | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.060 | 1.017–1.105 | 0.006 |
| Weight | 1.124 | 1.034–1.221 | 0.006 |
| BMI | 1.280 | 0.947–1.732 | 0.108 |
| Marital history | 1.000 | 0.192–5.202 | 1.000 |
| Fertility circumstance | 1,066,216,577.857 | 0.000 | 0.999 |
| Family history | 0.604 | 0.036–9.992 | 0.725 |
| Surgical option | 0.674 | 0.273–1.662 | 0.391 |
| Tumor size | 0.741 | 0.381–1.440 | 0.376 |
| ALN | 0.858 | 0.410–1.797 | 0.685 |
| Tumor grade | 1.429 | 0.593–3.440 | 0.426 |
| HER-2 | 1.936 | 0.675–5.558 | 0.219 |
| Ki-67 | 0.981 | 0.964–0.998 | 0.031 |
| P53 | 0.997 | 0.985–1.009 | 0.631 |
| P63 | 1.433 | 0.351–5.850 | 0.616 |
AR, androgen receptor; TNBC, triple-negative breast cancer; BMI, body mass index; ALN, axillary lymph node; HER-2, human epidermal growth factor receptor 2; OR, odds radio; CI, confidence interval.
Factors affecting TNBC prognosis
As of December 2021, after a median follow-up time of 37 months (range, 24–60 months), 13 (14.9%) relapse and metastasis cases were observed. There were 2 (15.4%) cases in the AR-positive group and 11 (84.6%) cases in the AR-negative group. The results for recurrence and metastasis in patients after TNBC surgery are set out in Table 3.
Table 3
| Groups | Recurrences and metastases (%) | Middle time (M, m) | Metastatic sites (n, %) |
|---|---|---|---|
| AR positive | 2 (15.4) | 44.5 | Lung (2, 100.0) |
| AR negative | 11 (84.6) | 19.0 | Lung (7, 63.6) |
| Liver (1, 9.1) | |||
| Bones (2, 18.2) | |||
| Chest wall (1, 9.1) |
TNBC, triple-negative breast cancer; AR, androgen receptor; M, median time; m, months.
The Cox regression analysis showed that relapse and metastasis was correlated with being unmarried (P=0.004; HR =0.105; 95% CI: 0.023–0.487), nonporous (P=0.046; HR =0.209; 95% CI: 0.045–0.971), and negative AR expression (P=0.042; HR =1.223; 95% CI: 0.049–1.012), while age, weight, BMI, family history, surgical option, tumor size, ALN status, tumor grade, Ki-67, P53, P63, chemotherapy, and radiotherapy did not affect prognosis. The logistic regression modeling results are presented in Table 4.
Table 4
| Characteristics | HR | 95% CI | P value |
|---|---|---|---|
| Age | 0.981 | 0.940–1.025 | 0.394 |
| Weight | 0.933 | 0.853–1.020 | 0.126 |
| BMI | 0.735 | 0.519–1.041 | 0.083 |
| Marital history | 0.105 | 0.023–0.487 | 0.004 |
| Fertility circumstance | 0.209 | 0.045–0.971 | 0.046 |
| Family history | 21.022 | 0.000–54,072,128.997 | 0.686 |
| Surgical option | 2.792 | 0.910–8.567 | 0.073 |
| Tumor size | 1.350 | 0.562–3.241 | 0.502 |
| ALN status | 0.668 | 0.207–2.152 | 0.499 |
| Tumor grade | 1.498 | 0.489–4.592 | 0.480 |
| Chemotherapy | 0.874 | 0.113–6.726 | 0.897 |
| Radiotherapy | 0.363 | 0.121–1.083 | 0.069 |
| Ki-67 | 1.012 | 0.989–1.036 | 0.295 |
| AR expression | 1.223 | 0.049–1.012 | 0.042 |
| P53 | 1.009 | 0.993–1.026 | 0.279 |
| P63 | 0.595 | 0.076–4.662 | 0.621 |
TNBC, triple-negative breast cancer; BMI, body mass index; ALN, axillary lymph node; AR, androgen receptor; HR, hazard radio; CI, confidence interval.
DFS and AR expression
We analyzed the DFS of the TNBC patients 2–5 years after surgery using Kaplan-Meier survival estimates. The results are shown in Figure 4. The postoperative DFS rates of the two groups at 2, 3, 4, and 5 years are shown in Table 5. The respective postoperative DFS rates for the AR-positive group and the AR-negative group at 2 years were 100.00% and 85.25%, at 3 years were 100.00% and 82.09%, at 4 years were 95.00% and 73.29%, and at 5 years were 88.21% and 67.85%, and the differences were statistically significant (P<0.05). The overall DFS was 53.6 months for the TNBC patients followed-up to 5 years after surgery. The overall survival (OS) of patients was 58.2 months in the AR-positive group and 50.6 months in the AR-negative group. The 5-year postoperative DFS of patients in the AR-positive group was better than that of patients in the AR-negative group, and the difference between the two groups was statistically significant (P<0.05).
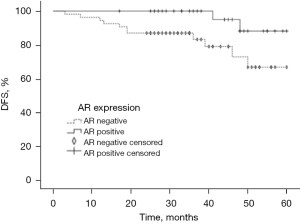
Table 5
| Group | n | 2 years* | 3 years* | 4 years* | 5 years* |
|---|---|---|---|---|---|
| AR positive | 33 | 100.00 | 100.00 | 95.00 | 88.21 |
| AR negative | 54 | 85.25 | 82.09 | 73.29 | 67.85 |
*, the follow-up time after the operation. DFS, disease-free survival; AR, androgen receptor.
TCM formula and DFS
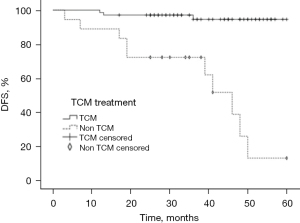
According to the statistical results, 67 (77.0%) TNBC patients took the Ruyan formula orally for >6 months after breast surgery. The median time for which patients took the TCM was 19 months (range, 6–57 months). As Figure 5 shows, the TCM formula improved the 2–5-year DFS of the TNBC patients after surgery (P<0.001).
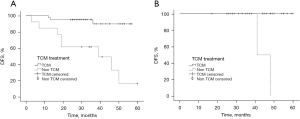
We conducted a subgroup analysis to examine the relationship between the TCM treatment and the DFS of TNBC patients with different AR expression levels (see Figure 6A,6B). In the AR-negative group, patients who received the TCM treatment tended to have better DFS than those who did not receive the TCM treatment (P<0.001; HR =10.738; 95% CI: 2.942–39.194). In the AR-positive group, the difference between the TCM treatment and the non-TCM treatment groups was not statistically significant.
Discussion
AR in TNBC
The application of AR as a prognostic marker of prostate cancer has been extensively explored in previous studies, and recently, more and more studies have investigated the application of AR in TNBC. In TNBC, ARs promote tumor progression to a certain extent (especially in the LAR subtype of TNBC) (18). ARs play an important role in breast development.
Research has shown that AR-knockout mice are characterized by reduced proliferation of mammary epithelial cells and reluctant ductal extension (19). Niță et al. (20) found that OS was improved by 2.1 months in the AR-positive group, compared to the AR-negative group. Venema et al. (21) analyzed the messenger ribonucleic acid (mRNA) profiles of 7,270 patients with primary breast tumors and concluded that a higher AR mRNA level was closely related to improving disease outcomes in ER-positive/HER2-negative patients, but was closely related to worse disease outcomes in HER2-positive patients. Qu et al. (22) showed in their meta-analysis of 3 studies specifically investigating TNBCs that AR expression was related to worse OS. Melo-Uribe et al. (23) found that TNBC patients with AR ≥50% had a younger age, lower Ki-67, and a median OS time of 2.45 years. Hu et al. (24) found that AR+ tumor patients are more likely to obtain a good prognosis for young, pre-menopausal, large tumor, more involved lymph nodes (4+), high stage, high grade, vascular invasion+, P53+, CK5/6− and Ki-67 patients. Their research shows that the absence of AR may help to identify patients with relatively high risk of disease recurrence and death. We found that TNBC patients with AR-positive expression were older in age, had lower Ki-67 levels, and better prognosis at our breast disease center.
This study had some limitations. First, our sample size was too small. Second, with the progress of clinical medicine, most breast cancer patients will not have recurrence and metastasis within 3 years. Thus, more studies with larger sample sizes and longer follow-up periods need to be conducted. Third, the optimal cut-off point for AR has not yet been determined. We used 1% as a cut-off point, but more experiments need to be conducted to demonstrate the standard of cut-off point for AR.
More and more research supports the conclusion that AR inhibitors, such as bicalutamide, and recent next-generation AR targeting agents, such as enzalutamide, abiraterone acetate, and seviteronel play a significant role in TNBC pathogenesis and may prove to be related to therapeutic targets for patients with AR positive expression breast cancer (25-27).
In a phase II study of enzalutamide (28), 118 TNBC patients were intent-to-treat (ITT) population, and their IHC reports for tumor tissue all had AR value >0%. Among these 118 patients, 78 had AR value ≥10% and formed the evaluable subgroup. Prior to disease progression, the patients received 160 mg of enzalutamide per day. The primary endpoint was the clinical benefit rate (CBR) at 16 weeks, and the secondary endpoints were the CBR at 24 weeks, progression-free survival (PFS), and safety. The patients had a median PFS time of 2.9 months in the ITT population and 3.3 months in the evaluable subgroup. There was 1 complete response in the evaluable subgroup at the 24-week CBR at, and 5 patients achieved a confirmed partial response. The median OS time was 12.7 months in the ITT population and 16.5 months in the evaluable subgroup. Fatigue was the only adverse event related to the treatment. Compared to the ITT population, the evaluable subgroup whose AR expression was ≥10% had better prognosis after treatment with enzalutamide.
In the TBCRC 032 IB/II Multicenter Study (29), the AR-positive TNBC patients received a treatment contained with enzalutamide (160 mg) and taselisib (4 mg). The treatment was stopped till disease progression. The TNBC patients whose subtype was LAR had a better response than those whose subtype was non-LAR (75.0% vs. 12.5%, P=0.06), and increased PFS (4.6 vs. 2.0 months, P=0.082). The adverse reactions included a skin rash and hyperglycemia.
In TBCRC011 (30,31), AR positive, ER/PR negative advanced breast cancer patients with bicalutamide performed 19% CBR at 6 months and a median PFS lasting 12 weeks. The results showed that anti-androgen receptor treatment was effective and had minimally toxic.
In addition to binding to androgens, ARs can also be stimulated in ligand-independent ways. ARs can be activated by interacting with important signaling pathways, such as the phosphatidylin-ositol-3-kinase (PI3K)/AKT/mTOR and canonical Wnt/β-catenin (Wnt/β-catenin) pathways (32). It is well known that the classical signaling pathway PI3K/AKT/mTOR is closely related to the development of breast cancer, including TNBC (33). In the subtype of LAR, the PIK3CA mutation was more significant in the AR-positive tumors than the AR-negative tumors. It was further found that enhancing the activity of PIK3CA protein in AR-positive TNBC directly activated the PI3K pathway without the aid of AR-mediated gene of phosphate and tension homology deleted on chromosome ten (PTEN)-upregulation (34). In non-LAR subtypes, there was evidence that AR could activate the epithelial growth factor receptor (EGFR) pathway by upregulating EGFR ligand amphiregulin level, thereby promoting cell proliferation (34). Thus, the dual targeting of AR and PI3K may have a synergistic antitumor effect (35).
In this research, we did not treat AR-positive TNBC patients with AR antagonists. Thus, the efficacy of AR antagonists in treating TNBC requires further attention (36). We also found that AR-positive expression was correlated with a higher weight in patients. Androgen excess is often associated with obesity (37). Obesity is an established risk factor for postmenopausal breast cancer. Obesity affects endogenous sex steroids and metabolic hormones, insulin sensitivity, and chronic inflammation (38). The presence of a hyperandrogenic state can also be detected in menopausal women, as a consequence of the rearrangement of the sex hormone balance, which in turn may play some role in determining the development of both visceral adiposity and even obesity and, consequently, metabolic disorders. Good living habits should be promoted and sedentary behavior avoided.
TCM in TNBC
The “Ruyan” formula contains many kinds of Chinese herbal medicines. The key ingredients are quercetin, luteolin, kaempferol, isorhamnetin and 7-Methoxy-2-methyl isoflavone (39). These ingredients act on anti-inflammatory, antioxidant, anti-tumor, anti-aging and other biological activities by regulating the activity of signal pathways and gene expression. They can also relieve cancer fatigue (40-42). In our previous research, we described the antitumor activity of TCM and natural medicine in TNBC based on different signaling pathways. It has been shown that TCM can inhibit the growth, proliferation, migration, invasion, and metastasis of TNBC cells through multiple pathways and targets, such as the PI3K/AKT/mTOR, Wnt/β-catenin, Signal Transducers and Activators of Transcription 3 (STAT3), and nuclear factor-kappa B (NF-KB) pathways (43).
Based on our findings, the TCM formula improved the prognosis of the TNBC patients. Under the guidance of an organic conception of the human body and syndrome differentiation, the correct and rational use of prescriptions and drugs can play a positive role in improving the symptoms of TNBC patients and preventing recurrence and metastasis. Due to the lack of effective treatment for TNBC, TCM can be used as a supplementary alternative therapy.
However, many patients did not adhere to the TCM treatment. This occurred for a number of reasons. First, many patients lived far away from the hospital and could not attend the hospital. Second, the travel of the patients was restricted due to Coronavirus Disease 2019. Thus, while it is useful, the pharmacology of the “Ruyan” formula is not yet clear.
Traditional Chinese medicine can rise to alleviate pain, improve living quality, prolong the sick role. However, different combinations and doses of drugs will produce different effects. A large number of studies are still needed to prove the pharmacological mechanism of traditional Chinese medicine recipe.
Conclusions
ARs are not generally highly expressed in TNBC patients. The AR-positive rate was only 37.9% at our hospital. TNBC patients with positive AR tended to have low expression of Ki-67 and a better prognosis. Given the limitations in relation to the therapy for TNBC, AR and TCM deserve more attention. The TCM formula could become a useful supplementary treatment for TNBC patients.
Acknowledgments
Funding: This research was supported by the National Natural Science Foundation of China (No. 82074438).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-508/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-508/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-508/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by EC (ethics committee) of The First Affiliated Hospital of Zhejiang Chinese Medical University (No. 2016-KL-020-02) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer 2007;110:876-84. [Crossref] [PubMed]
- Park IH, Yang HN, Jeon SY, et al. Anti-tumor activity of BET inhibitors in androgen-receptor-expressing triple-negative breast cancer. Sci Rep 2019;9:13305. [Crossref] [PubMed]
- Lian J, Ma HX, Xu EW, et al. Subclassifying triple-negative breast cancers and its potential clinical utility. Virchows Arch 2022;481:13-21. [Crossref] [PubMed]
- Jovanović B, Sheng Q, Seitz RS, et al. Comparison of triple-negative breast cancer molecular subtyping using RNA from matched fresh-frozen versus formalin-fixed paraffin-embedded tissue. BMC Cancer 2017;17:241. [Crossref] [PubMed]
- Lehmann BD, Jovanović B, Chen X, et al. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS One 2016;11:e0157368. [Crossref] [PubMed]
- Bou Zerdan M, Ghorayeb T, Saliba F, et al. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers (Basel) 2022;14:1253. [Crossref] [PubMed]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science 1988;240:889-95. [Crossref] [PubMed]
- Narayanan R, Coss CC, Dalton JT. Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol 2018;465:134-42. [Crossref] [PubMed]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 1994;63:451-86. [Crossref] [PubMed]
- Rampurwala M, Wisinski KB, O'Regan R. Role of the androgen receptor in triple-negative breast cancer. Clin Adv Hematol Oncol 2016;14:186-93. [PubMed]
- Wang W, Wu J, Zhang P, et al. Prognostic and predictive value of Ki-67 in triple-negative breast cancer. Oncotarget 2016;7:31079-87. [Crossref] [PubMed]
- Sharma P. Update on the Treatment of Early-Stage Triple-Negative Breast Cancer. Curr Treat Options Oncol 2018;19:22. [Crossref] [PubMed]
- Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA 2019;321:288-300. [Crossref] [PubMed]
- Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486:346-52. [Crossref] [PubMed]
- Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-67. [Crossref] [PubMed]
- Chan JJ, Tan TJY, Dent RA. Novel therapeutic avenues in triple-negative breast cancer: PI3K/AKT inhibition, androgen receptor blockade, and beyond. Ther Adv Med Oncol 2019;11:1758835919880429. [Crossref] [PubMed]
- Pietri E, Conteduca V, Andreis D, et al. Androgen receptor signaling pathways as a target for breast cancer treatment. Endocr Relat Cancer 2016;23:R485-98. [Crossref] [PubMed]
- Yeh S, Hu YC, Wang PH, et al. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J Exp Med 2003;198:1899-908. [Crossref] [PubMed]
- Niță I, Nițipir C, Toma ȘA, et al. Correlation between Androgen Receptor Expression and Immunohistochemistry Type as Prognostic Factors in a Cohort of Breast Cancer Patients: Result from a Single-Center, Cross Sectional Study. Healthcare (Basel) 2021;9:277. [Crossref] [PubMed]
- Venema CM, Bense RD, Steenbruggen TG, et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol Ther 2019;200:135-47. [Crossref] [PubMed]
- Qu Q, Mao Y, Fei XC, et al. The impact of androgen receptor expression on breast cancer survival: a retrospective study and meta-analysis. PLoS One 2013;8:e82650. [Crossref] [PubMed]
- Melo-Uribe MA, Serrano-Gómez SJ, Abaunza Chagin MC. Androgen receptor expression and prognosis in Hispanic/Latino women with triple negative breast cancer. Rev Esp Patol 2022;55:96-104. [Crossref] [PubMed]
- Hu XQ, Chen WL, Ma HG, et al. Androgen receptor expression identifies patient with favorable outcome in operable triple negative breast cancer. Oncotarget 2017;8:56364-74. [Crossref] [PubMed]
- Gucalp A, Traina TA. The Androgen Receptor: Is It a Promising Target? Ann Surg Oncol 2017;24:2876-80. [Crossref] [PubMed]
- Loibl S, Müller BM, von Minckwitz G, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 2011;130:477-87. [Crossref] [PubMed]
- Wang C, Pan B, Zhu H, et al. Prognostic value of androgen receptor in triple negative breast cancer: A meta-analysis. Oncotarget 2016;7:46482-91. [Crossref] [PubMed]
- Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol 2018;36:884-90. [Crossref] [PubMed]
- Lehmann BD, Abramson VG, Sanders ME, et al. TBCRC 032 IB/II Multicenter Study: Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR+ Metastatic Triple-Negative Breast Cancer. Clin Cancer Res 2020;26:2111-23. [Crossref] [PubMed]
- Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res 2013;19:5505-12. [Crossref] [PubMed]
- Gucalp A, Tolaney SM, Isakoff SJ, et al. TBCRC 011: Targeting the androgen receptor (AR) for the treatment of AR+/ER-/PR- metastatic breast cancer (MBC). J Clin Oncol 2011;29:TPS122. [Crossref]
- Anestis A, Zoi I, Papavassiliou AG, et al. Androgen Receptor in Breast Cancer-Clinical and Preclinical Research Insights. Molecules 2020;25:358. [Crossref] [PubMed]
- Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat 2018;169:397-406. [Crossref] [PubMed]
- Chen M, Yang Y, Xu K, et al. Androgen Receptor in Breast Cancer: From Bench to Bedside. Front Endocrinol (Lausanne) 2020;11:573. [Crossref] [PubMed]
- Bianchini G, De Angelis C, Licata L, et al. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol 2022;19:91-113. [Crossref] [PubMed]
- You CP, Leung MH, Tsang WC, et al. Androgen Receptor as an Emerging Feasible Biomarker for Breast Cancer. Biomolecules 2022;12:72. [Crossref] [PubMed]
- Pasquali R, Oriolo C. Obesity and Androgens in Women. Front Horm Res 2019;53:120-34. [Crossref] [PubMed]
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biologic mechanisms. Mol Oncol 2021;15:790-800. [Crossref] [PubMed]
- Cui Y, Mi J, Feng Y, et al. Huangqi Sijunzi decoction for treating cancer-related fatigue in breast cancer patients: a randomized trial and network pharmacology study. Nan Fang Yi Ke Da Xue Xue Bao 2022;42:649-57. [PubMed]
- Chen X, Liang D, Huang Z, et al. Anti-fatigue effect of quercetin on enhancing muscle function and antioxidant capacity. J Food Biochem 2021;45:e13968. [Crossref] [PubMed]
- Ganai SA, Sheikh FA, Baba ZA, et al. Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother Res 2021;35:3509-32. [Crossref] [PubMed]
- Tsai KJ, Tsai HY, Tsai CC, et al. Luteolin Inhibits Breast Cancer Stemness and Enhances Chemosensitivity through the Nrf2-Mediated Pathway. Molecules 2021;26:6452. [Crossref] [PubMed]
- Yang Z, Zhang Q, Yu L, et al. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J Ethnopharmacol 2021;264:113249. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)





