Breast reconstruction after mastectomy: does it decrease depression at the long-term?
Introduction
With a life-time risk of 12–13%, breast cancer is the most prevalent malignancy among Dutch women (1). Although mastectomy without breast reconstruction (M) is a very effective and often curative procedure, it has a profound mutilating effect that inevitably influences a patient psychologically, often causing not only negative body image and anxiety, but also depression (2,3), a condition that is associated with breast cancer survivors in 22% (range, 17–48%) according to the Beck Depression Inventory (BDI) (4). This high prevalence emphasizes the interest of research in this population. A recent study by Vodermaier et al. showed that depression is strongly associated with mortality in younger patients with early stage breast cancer (5). This is in agreement with Watson and colleagues (6), revealing that women with a high score on the Hospital Anxiety and Depression (HAD) scale category of depression had a significantly increased risk of death from all causes within 5 years (hazard ratio: 3.59; 95% CI: 1.39–9.24). Another study found a hazard ratio for all-cause mortality in breast cancer patients of 1.27 (95% CI: 0.58–2.79) (7).
After mastectomy, most women are given the option of either immediate (IBR) or delayed (DBR) breast reconstruction. Although these procedures are intended to provide cosmetic and psychological improvements, such as possible reduction of depression when compared to mastectomy without breast reconstruction (BR), contrasting reports have been written about these improvements. Multiple studies noted a significant decrease in depression in the reconstruction group (8-12), whereas in a number of other studies no psychological improvements were found in both IBR and DBR groups during follow-up periods of 6 months to 2 years after surgery (13,14). In view of the clinical possible consequences of BR, such as prolonged recovery time, risks of complications or the need of additional surgery, data should be available regarding the advantages of these surgical procedures including the influence on depression risk.
Since the combined data of previous studies are not only inconclusive, but also lack long-term follow-up, more research is needed on the effect of post-mastectomy BR on the long-term risk and severity of depression. In order to optimize pre-operative education and patient selection for BR, we evaluated the impact of BR on depression at the long-term, using the 13-item BDI (BDI-13).
Methods
Study design and study population
A single-institute cohort study was performed by using hospital databases. Only women who underwent curative mastectomy for breast cancer without relapse were included in this study. Males, patients who underwent other surgical operations than mastectomy, such as breast conserving surgery (BCS), patients with distant metastasis, ipsilateral relapse and patients who deceased for every kind of cause were excluded.
Procedures and techniques
Data collection
Included women received a study package by post containing an information letter describing both design and purpose of the study, a questionnaire covering general personal questions followed by the BDI-13 and a pre-addressed envelope. Patients were asked to voluntarily and anonymously participate and give informed consent by completing and returning the questionnaire. In order to collect baseline variables, questions were formulated based on personal and medical factors which may influence a patient’s psychological outcome (15). Only the medical researcher knew which study number belonged to which patient. The local ethical committee provided approval for this study (14u.003).
Measures
Obtained data included age, follow-up period, nationality, household type, children, educational level, body mass index (BMI), comorbidities, medication use and adjuvant therapies, which were collected from hospital records. Follow-up period was registered as time in years between the dates of mastectomy and participating in the study. Nationality was divided in Dutch or non-Dutch; type of household in living with or without a partner. It was registered whether patients did or did not have children. Educational level was classified using United Nations Educational, Scientific and Cultural Organization (UNESCO) International Standard Classification of Education (ISCED), in low (ISCED levels 1–2), intermediate (ISCED level 3) or high (ISCED levels 4–5) (16). BMI was both used as continuous variable as dichotomous variable i.e., low/normal weight or overweight/obese. The presences of comorbidities, such as hypertension, diabetes, chronic obstructive pulmonary disease were registered. Medication use was registered as polypharmacy, which defines usage of ≥4 types of medication, and/or the use of psychopharmacological agents such as hypnotics, sedatives, anxiolytics, antipsychotics, antidepressants and psychostimulants. Finally, it was registered whether patients underwent radiation therapy, chemotherapy, hormonal/endocrine therapy or immunotherapy.
The BDI-13 questionnaire is one of the most widely used screening tools for depression. It is developed to rate the severity of depressive symptoms (17) and exists of 13 clusters of 4 statements, ranking from 0 to 3, increasing in depression symptoms; in addition, a total score of 0–3 indicates minimal depression, 4–11 mild depression, 12–16 moderate depression and ≥17 severe depression. This questionnaire is accurate for somatic patients (18) and has been validated in many samples including cancer patients (19). Since no different cut off points indicating depression after breast cancer diagnosis are described in literature, the cut off values of the BDI-13 as stated above were used. Thus, scores of 0–3 and 4 or more is threshold for the absence or presence of depression, respectively. Comparison of baseline variables of these two study groups are shown in Table 1. Due to small numbers in almost all comorbidity groups these variables were pooled within the new variable comorbidity yes/no. Medication use was split in either polypharmacy or psychopharmacy due to small numbers of patients counted in the variable including both medication categories.
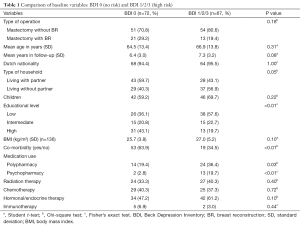
Full table
Statistical analysis
Descriptive and inferential statistics were used for analysis. All data were first tested for normality by a Q-Q plot, a Kolmogorov-Smirnov and a Levene’s test. Descriptive statistics were used to outline characteristics of included patients. Continuous normally distributed variables were expressed by their mean and standard deviation, not normally distributed data by their median and range. To test groups, categorical variables were tested using the Pearson’s Chi-square test of Fisher’s exact test, when appropriate. Normally distributed continuous data were tested with the independent samples Students t-test and in case of skewed data, with the independent samples Mann-Whitney U-test. Predictive variables for depression were found with multivariable logistic regression analysis, which was done through univariate logistic regression with all independent variables counting more than ten events. Variables showing P values <0.1 were then eligible for multivariable logistic regression. The maximum number of variables allowed in the multivariable model is the number of patients in the smallest group divided in ten. Significance level for baseline variables and multivariable regression analysis was set at P<0.05.
Results
A total of 1,074 patients were diagnosed with breast cancer in the Sint Lucas Andreas Hospital between 1999 and 2009. Of these 1,074 patients, 810 were primarily excluded as they underwent another surgical procedure than mastectomy, such as BCS, had a non-radical excision, metastasis, were treated elsewhere, were male, had a relapse or had deceased. A total of 264 women underwent a curative mastectomy and were therefore included in this study. Questionnaires were sent to these 264 women, of which nine were then excluded as they had recurrence of breast cancer or had deceased for any reason. An additional 62 women were unable or unwilling to fill out the questionnaire, due to old age (n=9), mental retardation (n=4), dementia (n=2), osteoarthrosis of the hands and other physical limitations restraining writing abilities (n=3), language-barriers (n=4), other reasons (n=3) or without stated reason (n=37). Another 50 women did not respond after repeated requests for participation (n=43) or were lost to follow-up (n=7). Since four returned questionnaires were incomplete and consequently excluded, the study population consisted of a total of 139 women, leading to a response rate of 52.7%. Informed consent was obtained from all individual participants included in the study.
The study population of 139 patients consisted of 105 (75.5%) participants in the M group and 34 (24.5%) in the mastectomy with breast reconstruction (MBR) group. Both groups had a long-term follow-up with the BDI questionnaire after mastectomy; 6.9 (SD: 3.2) and 6.5 (SD: 3.0) years in the M and MBR groups, respectively. Comparisons of baseline variables in M and MBR study groups are presented in Table 2. The M group had a mean age of 68.9 (SD: 12.8) years, whereas the MBR group had a mean age of 55.6 (SD: 10.9) years (P<0.01) at the time of this study. Of all women, 132 (95%) hold Dutch nationality. BR was performed more often in younger women and those who underwent adjuvant chemotherapy. Hypertension occurred more often in the M group. Other characteristics did not differ significantly between these groups.

Full table
BR and depression
The median BDI score of the entire study population (n=139) was 3 (range, 0–33). Within this population, a total of 67 (48.2%) patients were at high risk for depression, as ≥4 points were scored with the BDI-questionnaire. Of these 67 patients, 58 (86.6%) scored 4–11 points, indicating mild depression, 5 patients (7.5%) scored 12–16 points, indicating moderate depression and 4 patients (6%) scored ≥17 points, indicating severe depression.
The median BDI scores were 4 (range, 0–33) and 2 (range, 0–18) in the M and MBR group, respectively. No significant difference in BDI scores were found between these two groups (P=0.063). An additional analysis was done using the BDI score as dichotomous variable. Although this classification showed depression in 54 (51%) women in the M group and in 13 (38%) in the MBR group, no significant difference was found in both groups (P=0.18).
Other risk factors for depression
Univariately, the variables duration of follow-up, type of household, educational level, comorbidity, polypharmacy and adjuvant hormonal/endocrine therapy predicted a higher risk of depression (scores ≥4). Since the variables nationality, psychopharmacy and immunotherapy contained less than ten events, these variables were excluded from the logistic regression analysis. The continuous variables age and BMI, that showed no linear trend, were also excluded (Table 3).
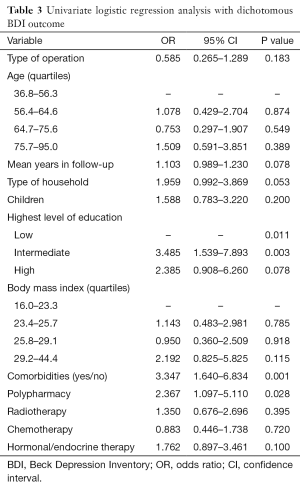
Full table
As polypharmacy contained the largest P value this variable was primarily excluded from de multivariable logistic regression. Although the next step would have been excluding either household type or educational level, both variables seemed to have an individual relationship with comorbidity (P<0.01). When these variables, which are confounders since a 10% ß difference was found, were corrected for interaction, no interaction in this multivariable model was found. Multivariable logistic regression analysis was done with variables household type, highest level of education and hormonal/endocrine therapy. Adjuvant hormonal therapy [odds ratio (OR): 2.36, P=0.02], living without a partner (OR: 2.16, P=0.04) and having a low educational level (OR: 3.7, P≤0.01) were associated with a higher risk of depression. Results of this multivariable logistic regression analysis are shown in Table 4.
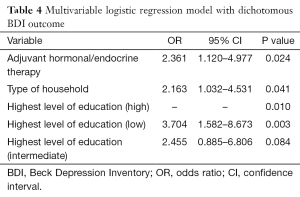
Full table
Discussion
In this study, no effect of BR on the BDI outcome was demonstrated, indicating that BR is not an effective therapy to reduce depression on the long-term. However, there was a slight trend towards a better BDI outcome for patients who underwent a BR after breast cancer related mastectomy. In literature, two prospective studies, analyzing depressive symptoms before and 1 year after surgery, showed no differences between patients who had undergone a mastectomy without reconstruction and patients with either immediate or delayed BR (20,21). In a cross-sectional study by Holly et al. (22), depressive symptoms were equal in the mastectomy with and without BR groups, whereas more depressive symptoms were found in the mastectomy without BR group in other studies (12,23). Likewise, contrasting reports have been published regarding quality of life (QoL), which was equal for patients with and without BR after mastectomy in several studies (13,24), whereas a better QoL and better psychological/emotional functioning was presented in the BR group in other reports (8,10,11).
When compared to literature (25), participants of this study showed high numbers of possible depression (48.2%) of which 41.7% may experience mild depression and 6.5% may experience moderate to severe depression. While the high incidence of depression among these patients imply that standard screening might be considered, the majority of patients at high risk for depression have a mild depression risk, whereas few patients are at high risk for moderate (n=5) to severe (n=4) depression. Although Watson et al. reported an increased risk of death in breast cancer women with a high score on the HAD scale category of depression within 5 years (hazard ratio: 3.59; 95% CI: 1.39–9.24), little is known about the dose response of severity of depression and survival. In order to improve survival by providing standard or selective screening, future research must investigate this clinically relevant topic.
Although data are available showing a significant relationship between depression and survival of breast cancer patients, findings are not consistent across studies (26). Further research concerning depression severity and survival is needed to give recommendations for selective or standard depression screening. Within current study population, a total of 15 patients used psychopharmacy, which included antidepressants within nine patients. The varying depression incidence in literature may be a result of different definitions of depression (25). Nevertheless, high prevalence of depression may be a result of major life events which trigger change processes in mental health, as an increase of depressive symptoms is not only seen in breast cancer diagnosis, but also in other cancer diagnosis (27). In a recently published large cohort study including 44.494 women with breast cancer, the ratio for a hospital contact for depression 1 year after surgery was 1.70 and that for the use of antidepressants 3.09. After 3 and 8 years, these ratios were significantly increased. Within this study, older age, living alone, basic educational levels and node-positive disease were associated with use of antidepressants. No association was found between type of surgery and the development of depression (28). Rubino et al. investigated depressive symptoms in women who had undergone a mastectomy and in healthy women, the latter showing fewer depressions (11).
In our study, patients who underwent BR were younger, had less hypertension, and had undergone adjuvant chemotherapy more often. All these findings are in accordance with data from literature (29). Household type, highest level of education and hormonal/endocrine therapy predicted higher risk of depression. These predictive values are consistent with literature where a married status showed a lower risk of depression when compared to a non-married status (30), lower social economic status (SES), that often includes low educational level, appears a predictor of developing depression (31-33) and depression is a known side-effect of hormonal therapy (34,35).
While this study found current results that are useful for the clinical setting, this study has several limitations. As it is not known which patients choose a BR, this introduced selection bias. There might not be an elevated risk of depression in the group that does not choose for a reconstruction. Taken this in mind, it could be hypothesized that patients with a high depression risk, do benefit from a BR. This should, however, be investigated in larger, prospective and longitudinal cohorts. Additionally, adding the influence of insurance companies might be an interesting topic for future studies.
In conclusion, this study found no significant effect of BR on the postoperative BDI-score; however, a slight trend was seen towards less depressive symptoms after BR. Future research in large and prospective longitudinal cohorts is needed to determine which variables and types of surgery influence breast cancer patients psychological outcome. Especially in woman who are single, have a low educational level and received adjuvant hormonal/endocrine therapy, awareness for depression is important.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The local ethical committee provided approval for this study (14u.003).
References
- Oncological care guideline. 2012. Available online: www.oncoline.nl/mammacarcinoom
- Gopie JP, ter Kuile MM, Timman R, et al. Impact of delayed implant and DIEP flap breast reconstruction on body image and sexual satisfaction: a prospective follow-up study. Psychooncology 2014;23:100-7. [Crossref] [PubMed]
- Christensen S, Zachariae R, Jensen AB, et al. Prevalence and risk of depressive symptoms 3-4 months post-surgery in a nationwide cohort study of Danish women treated for early stage breast-cancer. Breast Cancer Res Treat 2009;113:339-55. [Crossref] [PubMed]
- Zainal NZ, Nik-Jaafar NR, Baharudin A, et al. Prevalence of depression in breast cancer survivors: a systematic review of observational studies. Asian Pac J Cancer Prev 2013;14:2649-56. [Crossref] [PubMed]
- Vodermaier A, Linden W, Rnic K, et al. Prospective associations of depression with survival: a population-based cohort study in patients with newly diagnosed breast cancer. Breast Cancer Res Treat 2014;143:373-84. [Crossref] [PubMed]
- Watson M, Haviland JS, Greer S, et al. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet 1999;354:1331-6. [Crossref] [PubMed]
- Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. Gen Hosp Psychiatry 2006;28:396-402. [Crossref] [PubMed]
- Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg 2013;132:201e-209e. [Crossref] [PubMed]
- Bellino S, Fenocchio M, Zizza M, et al. Quality of life of patients who undergo breast reconstruction after mastectomy: effects of personality characteristics. Plast Reconstr Surg 2011;127:10-7. [Crossref] [PubMed]
- Fernández-Delgado J, López-Pedraza MJ, Blasco JA, et al. Satisfaction with and psychological impact of immediate and deferred breast reconstruction. Ann Oncol 2008;19:1430-4. [Crossref] [PubMed]
- Rubino C, Figus A, Lorettu L, et al. Post-mastectomy reconstruction: a comparative analysis on psychosocial and psychopathological outcomes. J Plast Reconstr Aesthet Surg 2007;60:509-18. [Crossref] [PubMed]
- Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer 2000;36:1938-43. [Crossref] [PubMed]
- Parker PA, Youssef A, Walker S, et al. Short-term and long-term psychosocial adjustment and quality of life in women undergoing different surgical procedures for breast cancer. Ann Surg Oncol 2007;14:3078-89. [Crossref] [PubMed]
- Sackey H, Sandelin K, Frisell J, et al. Ductal carcinoma in situ of the breast. Long-term follow-up of health-related quality of life, emotional reactions and body image. Eur J Surg Oncol 2010;36:756-62. [Crossref] [PubMed]
- Taira N, Shimozuma K, Shiroiwa T, et al. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat 2011;128:735-47. [Crossref] [PubMed]
- UNESCO. International Standard Classification of Education. Available online: https://webgate.ec.europa.eu/fpfis/mwikis/eurydice/index.php/Netherlands:Overview
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71. [Crossref] [PubMed]
- Furlanetto LM, Mendlowicz MV, Romildo Bueno J. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord 2005;86:87-91. [Crossref] [PubMed]
- Warmenhoven F, van Rijswijk E, Engels Y, et al. The Beck Depression Inventory (BDI-II) and a single screening question as screening tools for depressive disorder in Dutch advanced cancer patients. Support Care Cancer 2012;20:319-24. [Crossref] [PubMed]
- Metcalfe KA, Semple J, Quan ML, et al. Changes in psychosocial functioning 1 year after mastectomy alone, delayed breast reconstruction, or immediate breast reconstruction. Ann Surg Oncol 2012;19:233-41. [Crossref] [PubMed]
- Harcourt DM, Rumsey NJ, Ambler NR, et al. The psychological effect of mastectomy with or without breast reconstruction: a prospective, multicenter study. Plast Reconstr Surg 2003;111:1060-8. [Crossref] [PubMed]
- Holly P, Kennedy P, Taylor A, et al. Immediate breast reconstruction and psychological adjustment in women who have undergone surgery for breast cancer: a preliminary study. Psychol Health Med 2003;8:441-52. [Crossref] [PubMed]
- Pérez-San-Gregorio MA, Fernández-Jiménez E, Martín-Rodríguez A, et al. Quality of life in women following various surgeries of body manipulation: organ transplantation, mastectomy, and breast reconstruction. J Clin Psychol Med Settings 2013;20:373-82. [Crossref] [PubMed]
- Sun Y, Kim SW, Heo CY, et al. Comparison of quality of life based on surgical technique in patients with breast cancer. Jpn J Clin Oncol 2014;44:22-7. [Crossref] [PubMed]
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr 2004.57-71. [Crossref] [PubMed]
- Falagas ME, Zarkadoulia EA, Ioannidou EN, et al. The effect of psychosocial factors on breast cancer outcome: a systematic review. Breast Cancer Res 2007;9:R44. [Crossref] [PubMed]
- Infurna FJ, Gerstorf D, Ram N. The nature and correlates of change in depressive symptoms with cancer diagnosis: reaction and adaptation. Psychol Aging 2013;28:386-401. [Crossref] [PubMed]
- Suppli NP, Johansen C, Christensen J, et al. Increased risk for depression after breast cancer: a nationwide population-based cohort study of associated factors in Denmark, 1998-2011. J Clin Oncol 2014;32:3831-9. [Crossref] [PubMed]
- Parikh NI, Pencina MJ, Wang TJ, et al. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med 2008;148:102-10. [Crossref] [PubMed]
- Yan XY, Huang SM, Huang CQ, et al. Marital status and risk for late life depression: a meta-analysis of the published literature. J Int Med Res 2011;39:1142-54. [Crossref] [PubMed]
- Sajjadi H, Mohaqeqi Kamal SH, Rafiey H, et al. A systematic review of the prevalence and risk factors of depression among iranian adolescents. Glob J Health Sci 2013;5:16-27. [Crossref] [PubMed]
- Busch MA, Maske UE, Ryl L, et al. Prevalence of depressive symptoms and diagnosed depression among adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:733-9. [Crossref] [PubMed]
- Groffen DA, Koster A, Bosma H, et al. Unhealthy lifestyles do not mediate the relationship between socioeconomic status and incident depressive symptoms: the Health ABC study. Am J Geriatr Psychiatry 2013;21:664-74. [Crossref] [PubMed]
- Lorizio W, Wu AH, Beattie MS, et al. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat 2012;132:1107-18. [Crossref] [PubMed]
- Navari RM, Brenner MC, Wilson MN. Treatment of depressive symptoms in patients with early stage breast cancer undergoing adjuvant therapy. Breast Cancer Res Treat 2008;112:197-201. [Crossref] [PubMed]


