
Influence of vertical location and spacing of perforators on perfusion in deep inferior epigastric artery perforator flap breast reconstruction: quantitative analysis using indocyanine green angiography
Introduction
The deep inferior epigastric artery perforator (DIEP) flap has become a workhorse flap for autologous breast reconstruction, as it has the advantage of adequate tissue quantity, nearly ideal tissue characteristics, and low donor site morbidity (because it allows for preservation of the rectus abdominis muscle) (1,2). The number of perforators incorporated in the flap could be individualized according to the amount of tissue required for reconstruction and perforator characteristics, including size, orientation, eccentricity, and row. It is well known that enhanced flap perfusion positively correlates with the number of perforators (3-6). On the other hand, the degree of muscle resection becomes higher as more perforators are dissected to enhance flap perfusion, and donor site morbidity, such as bulging and herniation, could be increased when more perforators are harvested (7,8). Given that postoperative bulging or herniation could cause serious consequences—requiring further surgical intervention in some cases (9)—decisions on how many perforators are dissected for DIEP flaps should strike a balance between flap perfusion and donor site morbidity.
Regarding perforator location, several studies have compared perfusion patterns and vascular territory between medial- and lateral-row DIEP flap perforators. Anatomical vascular imaging studies have demonstrated that medial-row perforators preferentially perfuse the contralateral region of DIEP flaps with larger perfusion areas than those associated with lateral-row perforators. Lateral-row perforators preferentially perfuse the ipsilateral region with a smaller perfusion area (10-12). Medial-row perforators have been demonstrated to be associated with increased odds of fat necrosis in bilateral reconstructions (13). However, the influence of the vertical location of perforators on flap perfusion has rarely been assessed (14).
DIEP flaps are harvested from the lower abdomen to incorporate adequate amounts of soft tissue and to allow clothing to conceal abdominal scarring. On average, the dominant perforator is mostly located in the periumbilical region within 3-cm of the umbilicus (15). Therefore, most dominant perforators are located eccentrically in the upper portion of the flap. Additionally, when the dominant perforator is located beside the umbilical stalk, the umbilical incision can interfere with contralateral perfusion, reducing the perfused area, especially because linking vessels between perforators are oriented perpendicular to the midline of the trunk (16,17). We hypothesized that—relative to flaps with dominant perforators lower than the umbilical stalk—perfusion can be reduced in flaps with dominant perforators aligned with and above the umbilical stalk. To investigate this hypothesis, we quantitatively assessed DIEP flap perfusion, according to the vertical locations of perforators, using indocyanine green (ICG) angiography. The effect of the inclusion of additional caudal perforators [vertical spacing, as suggested by Lee et al. (14)] on flap perfusion was also quantitatively evaluated. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-371/rc).
Methods
Patients who underwent immediate or delayed, unilateral breast reconstruction with a DIEP flap between November 2018 and August 2021 by one of the authors (K.J.W. or J.W.P.), and were followed for a minimum of 6 postoperative months, were retrospectively identified from our prospectively collected database. Patients who underwent preoperative computed tomographic angiography and intraoperative ICG angiography were included. To maintain homogeneity of the study sample, we excluded patients who underwent simultaneous vascularized lymph node transfer using superficial circumflex iliac artery perforator flaps or if preoperative planning of bipedicled or multiple-perforator flap was conducted. Patients who had midline vertical abdominal scars were also excluded, as these scars can affect flap perfusion contralaterally to the pedicle. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (No. 2022-06-026), and informed consent was taken from all the patients.
Perfusion assessment using indocyanine green angiography
Surgical planning based on computed tomographic angiography was performed to estimate the flap inset rate and to identify perforators to be potentially harvested. The flap inset rate was defined as the proportion of inset flap to harvested flap weight. Mastectomy specimen weight and abdominal flap weight were estimated preoperatively using an integration method and a mobile application called “DIEP-W” as reported previously (18,19). Multiple-perforator flaps were planned for patients with no sizable perforators on computed tomographic angiography, and these patients were excluded from this study. Otherwise, the largest perforator on the preoperative computed tomographic angiography was identified and defined as the dominant perforator. Additional perforators to be potentially harvested were also selected that were less restricted by their locations and rows. Perforators located at or above the lower margin of the umbilical stalk were not selected as additional perforators according to their eccentric locations. In cases of immediate reconstruction, flap size was adjusted according to the actual mastectomy specimen weight after mastectomy. An estimate of inset rate was calculated intraoperatively using the mastectomy specimen weight and estimated abdominal flap weight. After the flap was designed and incised, targeted perforators were identified and isolated using bipolar electrocautery. The entry point of each perforator to the flap was marked on the skin surface using gentian violet.
Perfusion assessments using ICG angiography were performed in two stages. After all targeted perforators were isolated, the flap was entirely elevated from the abdominal wall. The first stage ICG angiography was performed after completion of intramuscular dissection of dominant perforator and cranial end (superior continuation) of the deep inferior epigastric vessels were ligated. Perforators except the dominant perforator were clamped using microvascular clamps to evaluate the proportion of the perfused area supplied solely by the dominant perforator. At the time of measurement, mean intra-arterial blood pressure was regulated between 70 and 90 mmHg and body temperature between 36 and 37 °C. The operating field was kept as dark as possible with a very dim light turned on for the anesthesiologists’ working area. After 10 mg of ICG (25 mg ICG mixed with 5 mL normal saline, Dongindang Pharm., Siheung, Korea) was administered, followed by a 10-mL saline flush, the perfused area of the flap was visualized using a hand-held, near-infrared camera (Fluobeam 800; Fluoptics, Grenoble, France). The area of earliest perfusion which was perfused in 30 to 40 s after fluorescence was started to visualize the abdominal flap was defined as ‘rapid perfusion area’ and was marked on the skin of the flap (20). The marked perfusion areas were at least 40% of fluorescence relative value units (RVUs) (Figure 1). RVU was defined as a percentage of fluorescence intensity relative to the surrounding well-perfused tissues designated as 100% fluorescent (21). After that, the microvascular clamps were removed, and complete pedicle dissection was performed. The second stage perfusion assessment was performed before pedicle division to evaluate effect of additional perforators on the perfusion zone. Perfusion assessment using ICG angiography was performed using the same method as the first stage. Total flap weight was measured after the pedicle division.
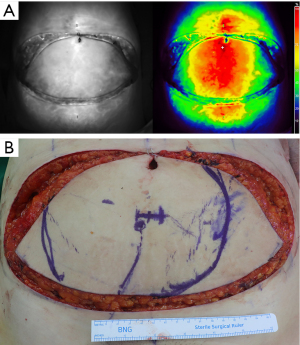
After performing microvascular anastomosis, we mostly discarded the soft tissue from the non-perfusion area (as determined by the perfusion assessment). We inset the flap temporarily, and further refinement of the flap was performed. The final inset volume was determined by comparing the mastectomy specimen weight and breast volumetry measured by computed tomographic angiography with the weight of the remaining flap tissue, which was calculated by subtracting the weight of the discarded flap tissue from the harvested flap weight.
Assignment of patients groups
Vertical perforator locations were classified into two zones: vertical zone 1 and vertical zone 2. Vertical zone 1 was defined as a perforator penetrating the anterior rectus sheath at or above the lower margin of the umbilical stalk on preoperative computed tomographic angiography. Vertical zone 2 was defined as a perforator penetrating the anterior rectus sheath below the lower margin of the umbilical stalk (Figure 2). Patients were categorized into two groups according to the vertical location of the dominant perforator. Patients who had a dominant perforator in vertical zone 1 and 2 were classified into cohorts 1 and 2, respectively.
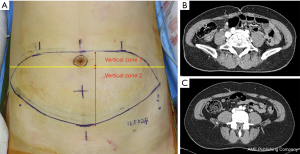
Outcome measurements
Outcomes of interest were perfused area, perfused proportion, maximal distance of midline cross, and perfusion-related complications (including fat necrosis and partial flap necrosis). The perfused proportion was defined as the perfused skin surface area divided by the total skin surface area of the harvested flap. The maximal distance of midline cross was defined as the longest distance from the midline of the flap to the contralateral perfusion (22). Each measurement was calculated using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Fat necrosis was defined as a >1-cm necrotic mass detected by physical examination or ultrasound. Ultrasound was performed by board-certified radiologists at 6 postoperative months and then regularly for cancer surveillance. Mastectomy skin flap necrosis was defined as any breakdown of skin integrity on the mastectomy site that was treated with surgical intervention.
Statistical analysis
Mean and standard deviations are used to summarize continuous variables; frequencies and proportions are used to describe categorical variables. Shapiro-Wilk tests were performed to determine distribution normality. Clinical and surgical variables were compared between the two groups using two-sample t-test or Mann-Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. The Statistical Package for the Social Sciences (SPSS version 21; IBM Co., Armonk, NY, USA) was used for data analysis. The statistical significance was indicated by P values <0.05.
Results
Among 101 patients who underwent unilateral breast reconstruction with DIEP flaps during the study period, 67 were included in this study (Figure 3). The mean patient age was 49.5±8.3 years (range, 27 to 71 years), and the mean body mass index was 24.2±3.1 kg/m2 (range, 18.1 to 33.1 kg/m2). Immediate reconstruction was performed in 49 patients and delayed reconstruction in 18. The median follow-up period was 14.5 months (range, 6 to 36 months). The incidence of fat necrosis was 4.5% (3 of 67 patients), including 9.5% in cohort 1 (2 of 21 patients) and 2.2% in cohort 2 (1 of 46 patients). No partial flap necrosis and three cases of mastectomy skin flap necrosis was found in the overall study population. Three patients were diagnosed as having fat necrosis by postoperative ultrasound, and two of the three patients had palpable masses on physical examination. In terms of clinical and surgical variables, there were significantly more harvested perforators and a lower inset rate in cohort 1 compared with cohort 2. Other clinical and surgical characteristics were similar between the two cohorts (Table 1).
Table 1
| Variable | Cohort 1* | Cohort 2† | P |
|---|---|---|---|
| No. of patients | 21 | 46 | |
| Age, years | 51.8±9.7 | 48.5±7.4 | 0.129 |
| BMI, kg/m2 | 24.3±3.5 | 24.1±3.0 | 0.829 |
| Comorbidities | |||
| Hypertension | 2 (9.5) | 3 (6.5) | 0.645 |
| Diabetes | 1 (4.8) | 1 (4.3) | 1.000 |
| Smoking | 0 | 4 (8.7) | 0.301 |
| Prior radiotherapy | 4 (19.0) | 7 (15.2) | 0.730 |
| Prior chemotherapy | 3 (14.3) | 14 (30.4) | 0.159 |
| Prior abdominal surgery | 0.408 | ||
| Pfannenstiel incision | 12 (57.1) | 18 (39.1) | |
| Laparoscopic incision | 2 (9.5) | 7 (15.2) | |
| Other incision | 1 (4.8) | 1 (2.2) | |
| No. of perforators | 2.1±0.5 | 1.5±0.6 | <0.001 |
| Row of dominant perforator | 1.000 | ||
| Medial | 17 (81.0) | 37 (80.4) | |
| Lateral | 4 (19.0) | 9 (19.6) | |
| Vertical height of flap, cm | 12.4±1.0 | 12.6±0.9 | 0.405 |
| Harvested flap weight, g | 735.3±298.6 | 698.5±250.0 | 0.601 |
| Inset weight, g | 390.7±126.8 | 420.8±142.5 | 0.409 |
| Inset rate | 0.55±0.10 | 0.62±0.12 | 0.022 |
Data are shown as mean ± standard deviation or number (percentage). *, patients who had a dominant perforator in vertical zone 1; †, patients who had a dominant perforator in vertical zone 2. BMI, body mass index.
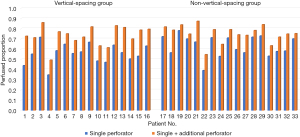
Outcome variables regarding perfusion by single dominant perforators were evaluated in the overall study population, including 21 patients in cohort 1 and 46 patients in cohort 2. The results are summarized in Table 2. The perfused area, perfused proportion, and maximal distance of midline cross were significantly greater in cohort 2 than cohort 1. Seventy percent of the flap area was perfused by single dominant perforators in cohort 2, while the perfused proportion was limited to 56 percent in cohort 1. Regardless of the vertical location of the dominant perforators, incorporation of additional perforator increased perfused proportions in all cases (Figure 4).
Table 2
| Variable | Cohort 1* | Cohort 2† | P |
|---|---|---|---|
| Perfused area, cm2 | 141.6±39.3 | 168.1±31.7 | 0.005 |
| Perfusion proportion | 0.56±0.09 | 0.70±0.09 | <0.001 |
| Maximal distance of midline cross, cm | 4.7±1.8 | 6.1±1.7 | 0.001 |
Data are shown as mean ± standard deviation. *, patients who had a dominant perforator in vertical zone 1; †, patients who had a dominant perforator in vertical zone 2.
Next, we evaluated the effects of vertical spacing of perforators on outcome variables. Patients who had one dominant perforator and one additional perforator were included in this further analysis. Patients with two or more additional perforators were excluded because the number of additional perforators can be a confounder. Given that all additional perforators were harvested from vertical zone 2, patients with dominant perforators in vertical zone 1 were classified into the vertical-spacing group in which perforators were included both in vertical zones 1 and 2. Patients with dominant perforators in vertical zone 2 were classified into the no-vertical-spacing group. Thirty-three patients were identified, including 16 patients in the vertical-spacing group and 17 patients in the no-vertical-spacing group. All additional perforators were located in the same row as the dominant perforator in each patient, except one patient in the vertical-spacing group who had the dominant perforator in the lateral row but the additional perforator in the medial row. Table 3 presents baseline clinical and surgical characteristics of the 33 patients. The two groups were similar in terms of clinical and surgical characteristics.
Table 3
| Variable | Vertical-spacing* group | No-vertical-spacing† group | P |
|---|---|---|---|
| No. of patients | 16 | 17 | |
| Age, years | 51.4±10.8 | 48.5±8.4 | 0.394 |
| BMI, kg/m2 | 24.6±3.9 | 23.8±3.1 | 0.638 |
| Comorbidities | |||
| Hypertension | 2 (12.5) | 0 | 0.227 |
| Diabetes | 1 (6.3) | 0 | 0.485 |
| Smoking | 0 | 0 | – |
| Prior radiotherapy | 2 (12.5) | 4 (23.5) | 0.656 |
| Neoadjuvant chemotherapy | 1 (6.3) | 5 (29.4) | 0.175 |
| Prior abdominal surgery | 0.288 | ||
| Pfannenstiel incision | 9 (56.3) | 6 (35.3) | |
| Laparoscopic incision | 2 (12.5) | 2 (11.8) | |
| Other incision | 1 (6.3) | 0 | |
| Row of dominant perforator | 1.000 | ||
| Medial | 12 (75.0) | 12 (70.6) | |
| Lateral | 4 (25.0) | 5 (29.4) | |
| Vertical height of flap, cm | 12.3±1.1 | 12.7±0.8 | 0.239 |
| Harvested flap weight, g | 742.5±343.3 | 715.5±206.8 | 0.784 |
| Inset weight, g | 386.9±140.5 | 432.5±109.7 | 0.304 |
| Inset rate | 54.7±11.3 | 61.8±10.5 | 0.069 |
Data are shown as mean ± standard deviation or number (percentage). *, a dominant perforator in vertical zone 1 and an additional perforator in vertical zone 2; †, both dominant and additional perforators in vertical zone 2. DIEP, deep inferior epigastric artery perforator; BMI, body mass index.
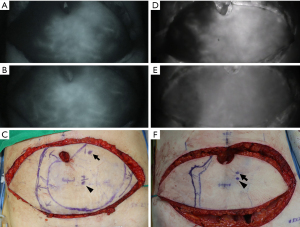
Additional perforators to the dominant perforator were associated with larger perfused areas in both the vertical-spacing and no-vertical-spacing groups, but the perfused area increment was significantly greater in the vertical-spacing group (43.4 vs. 29.4 cm2; P=0.009) (Table 4). Fifty-six percent of the total flap skin area was perfused by a dominant perforator in the vertical-spacing group, and the perfused proportion was increased to 73% by adding an additional perforator. In the no-vertical-spacing group, 63% of the total flap skin area was perfused by a dominant perforator, and the perfused proportion was increased to 75% by adding an additional perforator. The perfused proportion increment achieved by adding an additional perforator was significantly higher in the vertical-spacing group than in the no-vertical-spacing group (17% vs. 12%; P=0.004). The increments of the maximal distance of midline cross was also significantly larger in the vertical-spacing group compared with the no-vertical-spacing group (2.3 vs. 1.4 cm; P=0.039). Representative patients comparing the two groups are presented in Figure 5.
Table 4
| Variable | Vertical-spacing* group | No-vertical-spacing† group | P |
|---|---|---|---|
| Perfused area, cm2 | |||
| Dominant perforator | 140.6±42.9 | 154.7±27.5 | |
| All perforators | 184.0±48.4 | 184.1±28.9 | |
| Increment of dimension | 43.4±13.8 | 29.4±15.2 | 0.009 |
| Perfused proportion | |||
| Dominant perforator | 0.56±0.09 | 0.63±0.10 | |
| All perforators | 0.73±0.09 | 0.75±0.08 | |
| Increment of proportion | 0.17±0.05 | 0.12±0.05 | 0.004 |
| Maximal distance of midline cross, cm | |||
| Dominant perforator | 4.4±1.9 | 5.5±1.5 | |
| All perforators | 6.7±1.9 | 6.8±2.3 | |
| Increment of distance | 2.3±1.4 | 1.4±1.6 | 0.039 |
Data are shown as mean ± standard deviation. *, a dominant perforator in vertical zone 1 and an additional perforator in vertical zone 2; †, both dominant and additional perforators in vertical zone 2. DIEP, deep inferior epigastric artery perforator.
Discussion
This study evaluated the impact of vertical location and spacing of perforators on the perfusion of flaps in patients who underwent DIEP flap breast reconstruction. Perfusion-related outcome variables, including perfused area, perfused proportion, and maximal distance of midline cross were significantly higher in cohort 2 compared with cohort 1 in the evaluation of perfusion by single dominant perforators. Increments of perfusion-related outcome variables were significantly higher among patients who underwent vertical spacing of perforators compared with patients who did not undergo vertical spacing of perforators. These results indicated that a single dominant perforator in vertical zone 2 could perfuse a larger proportion of flap than in vertical zone 1, and vertical perforator spacing can increase the perfusion capacity of additional perforators included in the flap. These results could have significant impacts on clinical practice, considering that DIEP flap perforators are usually found near the umbilicus, so perforators to be harvested are determined between vertical zones 1 and 2 in most cases (15).
Despite the advantage of the lower incidence of abdominal wall morbidity associated with DIEP flaps compared with transverse rectus abdominis myocutaneous flaps, fat necrosis is a nonnegligible drawback of DIEP flaps (23). Clinical features of fat necrosis include a single lump, multiple round nodules, irregular masses with skin retraction, or incidental findings on imaging studies. Close follow-up or pathologic confirmation is necessary when suspicious imaging findings are observed, such as irregular masses or posterior acoustic shadowing on ultrasound, and rim enhancement on magnetic resonance imaging (24). Given that most patients who undergo DIEP flap reconstruction are cancer survivors, these clinical features of fat necrosis—mimicking cancer recurrence—can cause worry and serious psychological distress (25). Additionally, fat necrosis requiring reoperation can result in significant breast deformity and consequent emotional distress and increased medical costs. Recently, the use of ICG angiography in DIEP flap breast reconstruction has been demonstrated to be associated with a reduced risk of fat necrosis (26), and the incidence of fat necrosis has been shown to range from 8.3% to 29% among patients who have undergone intraoperative ICG angiography (27-31). Three studies have demonstrated intraoperative ICG administration to be associated with decreased rate of fat necrosis, and subjective assessment of ICG enhancement has been used to determine the area to be excised (28,29,31). On the other hand, Yoo et al. used objective measurements in ICG angiography. They discarded tissues that had RVUs less than 25% to 30% and found that no statistically significant differences were observed in the incidence of fat necrosis between patients assessed with ICG angiography and patients assessed clinically (30). There is still no strong consensus on objective measurements from ICG angiography to predict perfusion outcomes in abdomen-based breast reconstruction, but relative values have been demonstrated to be superior than absolute values because absolute values can be affected by patient-related variables, such as obesity, smoking, and diabetes (32,33).
Regarding evaluation timing using ICG angiography, we assessed flaps 30 to 40 s after fluorescence was started for abdominal flap visualization. Gorai et al. demonstrated that the time to reach half of maximal perfusion was a more sensitive variable than fluorescence intensity in their evaluation of perfusion of mastectomy skin flaps using ICG angiography (34). They demonstrated that necrotic skin area showed significantly longer time to reach half of maximal perfusion than viable area. Koonce et al. reported that delayed enhancement by ICG was more likely to be associated with fat necrosis in assessment of perfusion of extended transverse skin paddles in muscle-sparing latissimus dorsi flaps (20). We suggest that evaluation timing is a crucial component of perfusion assessment in DIEP flap breast reconstruction, and we were able to identify distinct illuminated areas about 30 to 40 s after fluorescence was started for abdominal flap visualization, which we termed the rapid perfusion area. This rapid perfusion area corresponded with areas with RVUs of 40% or higher, which was higher than the values suggested by Yoo et al. (30). We believe that the rapid perfusion area can be used as a threshold for a reliable perfusion zone, since our study population had a low incidence of perfusion-related complications, including a 4.5% incidence of fat necrosis and no cases of partial flap loss. In terms of flap volumes, the mean perfused proportions were 73% and 75% in the vertical-spacing and no-vertical-spacing groups, respectively, which could be acceptable inset rates for breast reconstruction.
Despite venous drainage is also an essential component in assessment of flap circulation, few studies have evaluated venous drainage using ICG angiography. Kurita et al. reported the dominant drainage vein could be detected using ICG angiography in a case of fingertip replantation (35). In our opinion, venous dominance between deep inferior epigastric vein and superficial inferior epigastric vein could also be assessed using ICG angiography and would be a good candidate for further research.
Flaps with centrally located perforators have been regarded as a safer option (36), in line with the angiosome theory described by Taylor et al. (37-39) and the perforasome theory suggested by Saint-Cyr et al. (16). When designing DIEP flaps for breast reconstruction, however, perforators are usually located eccentrically, according to the ideal position of flap design and periumbilical location of perforators (15). Kelly et al. demonstrated that umbilicus act as a physical barrier to paraumbilical perforators of DIEP flap and contralateral perfusion could be compromised when paraumbilical perforators were harvested (17). We set the horizontal line crossing the lower margin of umbilical stalk as the threshold of vertical perforator location because we assumed that this line can divide perforators into two groups that have quite different characteristics in terms of flap perfusion. A dominant perforator located above this line would be located eccentrically to the flap, and a considerable amount of contralateral perfusion could be blocked by an umbilical incision for flap elevation. Conversely, a dominant perforator located below this line would be located more centrally in the flap, and linking vessels to the contralateral side of the flap would be relatively well maintained. We consistently found that most of the contralateral paraumbilical area was not included in the rapid perfusion area in the first stage of perfusion assessment (for single dominant perforators in vertical zone 1), as depicted in Figure 5.
Our findings lend some important considerations to the preoperative planning of DIEP flap. In cases with dominant perforators in vertical zone 1 requiring large flaps, the inclusion of additional perforators in vertical zone 2 could be actively considered, or a bipedicled flap could be incorporated in the planning when suitable perforators are not found in vertical zone 2, as dominant perforators in vertical zone 1 are expected to have lower perfusion capacity compared with zone 2 perforators. Given that dominant perforators were found in vertical zone 1 in a considerable portion of the study population (31.3%), the results of this study could have a significant impact on preoperative planning for patients scheduled for DIEP flap reconstruction. Additionally, quantitative data regarding the perfused proportion in this study can help volumetric planning of the DIEP flap according to perforator locations, because inset rate for safe use of the flap can be estimated before surgery. If less than 60% of inset rate is necessary, single dominant perforator can be planned even though the perforator is located in vertical zone 1. If the dominant perforator is located in vertical zone 2, planning a single perforator DIEP flap can be more feasible considering a mean perfusion ratio of the flap was as high as 70% in this study. Unnecessary incorporation of multiple perforators for DIEP flap can increase complexity of the procedure and degree of rectus muscle injury without gain of perfusion related outcomes.
This study had several limitations, including its retrospective design. Relevant factors regarding perfusion-related clinical outcomes could not be evaluated, given the considerably low incidence of partial flap necrosis and fat necrosis. Additionally, quantitative analysis regarding the effects of perforator diameter on perfusion-related outcomes was not performed in this study. According to Poiseuille’s law, vessel diameter is a principal factor contributing to flap circulation (36). Preoperative evaluation of perforator diameter using a high-resolution imaging modality, such as ultrasound, may aid preoperative planning and improve perfusion-related outcomes (40). Similarly, the potential effect of previous Pfannenstiel incision on the perfusion-related outcome was not evaluated in this study (41). Further quantitative analysis of the effect of the Pfannenstiel incision on the perfusion-related outcome would be warranted. Another limitation was that the study population was limited to Korean women. The low mean body mass index and other racial characteristics could have biased the study findings. Lastly, mean intra-arterial blood pressure was regulated between 70 and 90 mmHg and body temperature between 36 and 37 °C in this study, but the differences of 20 mmHg and 1 °C still have potential to affect the flap perfusion.
Conclusions
DIEP flap perfusion can be affected by the vertical location of perforators, and flap perfusion can be augmented effectively by vertical spacing of perforators. Perfusion-related complications and donor site morbidity could be balanced by using the suggested protocol for perforator selection and flap design.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-371/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-371/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-371/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-371/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (No. 2022-06-026), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-5. [Crossref] [PubMed]
- Momeni A, Sheckter C. Intraoperative Laser-Assisted Indocyanine Green Imaging Can Reduce the Rate of Fat Necrosis in Microsurgical Breast Reconstruction. Plast Reconstr Surg 2020;145:507e-13e. [Crossref] [PubMed]
- Mulvey CL, Cooney CM, Daily FF, et al. Increased Flap Weight and Decreased Perforator Number Predict Fat Necrosis in DIEP Breast Reconstruction. Plast Reconstr Surg Glob Open 2013;1:1-7. [Crossref] [PubMed]
- Baumann DP, Lin HY, Chevray PM. Perforator number predicts fat necrosis in a prospective analysis of breast reconstruction with free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg 2010;125:1335-41. [Crossref] [PubMed]
- Grover R, Nelson JA, Fischer JP, et al. The impact of perforator number on deep inferior epigastric perforator flap breast reconstruction. Arch Plast Surg 2014;41:63-70. [Crossref] [PubMed]
- Bozikov K, Arnez T, Hertl K, et al. Fat necrosis in free DIEAP flaps: incidence, risk, and predictor factors. Ann Plast Surg 2009;63:138-42. [Crossref] [PubMed]
- Butler DP, Plonczak AM, Reissis D, et al. Factors that predict deep inferior epigastric perforator flap donor site hernia and bulge. J Plast Surg Hand Surg 2018;52:338-42. [Crossref] [PubMed]
- Hembd A, Teotia SS, Zhu H, et al. Optimizing Perforator Selection: A Multivariable Analysis of Predictors for Fat Necrosis and Abdominal Morbidity in DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2018;142:583-92. [Crossref] [PubMed]
- Mennie JC, Mohanna PN, O'Donoghue JM, et al. Donor-Site Hernia Repair in Abdominal Flap Breast Reconstruction: A Population-Based Cohort Study of 7929 Patients. Plast Reconstr Surg 2015;136:1-9. [Crossref] [PubMed]
- Wong C, Saint-Cyr M, Mojallal A, et al. Perforasomes of the DIEP flap: vascular anatomy of the lateral versus medial row perforators and clinical implications. Plast Reconstr Surg 2010;125:772-82. [Crossref] [PubMed]
- Wong C, Saint-Cyr M, Arbique G, et al. Three- and four-dimensional computed tomography angiographic studies of commonly used abdominal flaps in breast reconstruction. Plast Reconstr Surg 2009;124:18-27. [Crossref] [PubMed]
- Schaverien M, Saint-Cyr M, Arbique G, et al. Arterial and venous anatomies of the deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg 2008;121:1909-19. [Crossref] [PubMed]
- Kamali P, Lee M, Becherer BE, et al. Medial Row Perforators Are Associated with Higher Rates of Fat Necrosis in Bilateral DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2017;140:19-24. [Crossref] [PubMed]
- Lee KT, Eom Y, Jeon BJ, et al. Vertical Spacing of Perforators in Deep Inferior Epigastric Perforator Flap Breast Reconstruction Can Affect the Outcomes. Plast Reconstr Surg 2018;142:319-29. [Crossref] [PubMed]
- Bailey SH, Saint-Cyr M, Wong C, et al. The single dominant medial row perforator DIEP flap in breast reconstruction: three-dimensional perforasome and clinical results. Plast Reconstr Surg 2010;126:739-51. [Crossref] [PubMed]
- Saint-Cyr M, Wong C, Schaverien M, et al. The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg 2009;124:1529-44. [Crossref] [PubMed]
- Kelly JA, Pacifico MD. Lateralising paraumbilical medial row perforators: dangers and pitfalls in DIEP FLAP planning: a systematic review of 1116 DIEP flaps. J Plast Reconstr Aesthet Surg 2014;67:383-8. [Crossref] [PubMed]
- Woo KJ, Kim EJ, Lee KT, et al. A Novel Method to Estimate the Weight of the DIEP Flap in Breast Reconstruction: DIEP-W, a Simple Calculation Formula Using Paraumbilical Flap Thickness. J Reconstr Microsurg 2016;32:520-7. [Crossref] [PubMed]
- Kim H, Lim SY, Pyon JK, et al. Preoperative computed tomographic angiography of both donor and recipient sites for microsurgical breast reconstruction. Plast Reconstr Surg 2012;130:11e-20e. [Crossref] [PubMed]
- Koonce SL, Barnavon Y, Newman MI, et al. Perfusion Zones of Extended Transverse Skin Paddles in Muscle-Sparing Latissimus Dorsi Myocutaneous Flaps for Breast Reconstruction. Plast Reconstr Surg 2019;143:920e-6e. [Crossref] [PubMed]
- Griffiths M, Chae MP, Rozen WM. Indocyanine green-based fluorescent angiography in breast reconstruction. Gland Surg 2016;5:133-49. [Crossref] [PubMed]
- Han HH, Kang MK, Choe J, et al. Estimation of Contralateral Perfusion in the DIEP Flap by Scoring the Midline-Crossing Vessels in Computed Tomographic Angiography. Plast Reconstr Surg 2020;145:697e-705e. [Crossref] [PubMed]
- Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg 2000;106:576-83. [Crossref] [PubMed]
- Tayyab SJ, Adrada BE, Rauch GM, et al. A pictorial review: multimodality imaging of benign and suspicious features of fat necrosis in the breast. Br J Radiol 2018;91:20180213. [Crossref] [PubMed]
- Peeters WJ, Nanhekhan L, Van Ongeval C, et al. Fat necrosis in deep inferior epigastric perforator flaps: an ultrasound-based review of 202 cases. Plast Reconstr Surg 2009;124:1754-8. [Crossref] [PubMed]
- Lauritzen E, Damsgaard TE. Use of Indocyanine Green Angiography decreases the risk of complications in autologous- and implant-based breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2021;74:1703-17. [Crossref] [PubMed]
- Malagón-López P, Carrasco-López C, García-Senosiain O, et al. When to assess the DIEP flap perfusion by intraoperative indocyanine green angiography in breast reconstruction? Breast 2019;47:102-8. [Crossref] [PubMed]
- Malagón-López P, Vilà J, Carrasco-López C, et al. Intraoperative Indocyanine Green Angiography for Fat Necrosis Reduction in the Deep Inferior Epigastric Perforator (DIEP) Flap. Aesthet Surg J 2019;39:NP45-54. [Crossref] [PubMed]
- Hembd AS, Yan J, Zhu H, et al. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast Reconstr Surg 2020;146:1e-10e. [Crossref] [PubMed]
- Yoo A, Palines PA, Mayo JL, et al. The Impact of Indocyanine Green Angiography on Fat Necrosis in Deep Inferior Epigastric Perforator Flap Breast Reconstruction. Ann Plast Surg 2022;88:415-9. [Crossref] [PubMed]
- Varela R, Casado-Sanchez C, Zarbakhsh S, et al. Outcomes of DIEP Flap and Fluorescent Angiography: A Randomized Controlled Clinical Trial. Plast Reconstr Surg 2020;145:1-10. [Crossref] [PubMed]
- Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg 2012;129:1043-8. [Crossref] [PubMed]
- Fourman MS, Gersch RP, Levites HA, et al. Is There a Right Way to Interpret SPY? Normalization of Indocyanine Green Angiography Readings in a Burn Model. Plast Reconstr Surg 2015;136:128e-30e. [Crossref] [PubMed]
- Gorai K, Inoue K, Saegusa N, et al. Prediction of Skin Necrosis after Mastectomy for Breast Cancer Using Indocyanine Green Angiography Imaging. Plast Reconstr Surg Glob Open 2017;5:e1321. [Crossref] [PubMed]
- Kurita M, Shiraishi T, Ozaki M, et al. Usefulness of microscope-based ICG videoangiography for detection of the dominant drainage vein in fingertip replantation. J Plast Reconstr Aesthet Surg 2010;63:2200-1. [Crossref] [PubMed]
- Blondeel PN. Discussion: perfusion-related complications are similar for DIEP and muscle-sparing free TRAM flaps harvested on medial or lateral deep inferior epigastric artery branch perforators for breast reconstruction. Plast Reconstr Surg 2011;128:590e-592e. [Crossref] [PubMed]
- Taylor GI, Corlett RJ, Dhar SC, et al. The anatomical (angiosome) and clinical territories of cutaneous perforating arteries: development of the concept and designing safe flaps. Plast Reconstr Surg 2011;127:1447-59. [Crossref] [PubMed]
- Taylor GI, Chubb DP, Ashton MW. True and 'choke' anastomoses between perforator angiosomes: part i. anatomical location. Plast Reconstr Surg 2013;132:1447-56. [Crossref] [PubMed]
- Chubb DP, Taylor GI, Ashton MW. True and 'choke' anastomoses between perforator angiosomes: part II. dynamic thermographic identification. Plast Reconstr Surg 2013;132:1457-64. [Crossref] [PubMed]
- Homsy C, McCarthy ME, Lim S, et al. Portable Color-Flow Ultrasound Facilitates Precision Flap Planning and Perforator Selection in Reconstructive Plastic Surgery. Ann Plast Surg 2020;84:S424-30. [Crossref] [PubMed]
- Mahajan AL, Zeltzer A, Claes KEY, et al. Are Pfannenstiel scars a boon or a curse for DIEP flap breast reconstructions? Plast Reconstr Surg 2012;129:797-805. [Crossref] [PubMed]



