
Developing and validating a nomogram to predict myasthenia gravis exacerbation in patients with postoperative thymoma recurrence
Introduction
Thymoma is a rare and potentially malignant tumor, and complete surgical resection is currently recognized globally as the first choice of treatment (1). Nevertheless, 10–30% of patients may have postoperative recurrence (2-4). Myasthenia gravis (MG) is an acquired neuromuscular junction (NMJ) transmission disorder caused by autoantibodies (5), and MG patients often have accompanying thymic abnormalities, including thymic hyperplasia, thymic atrophy, and thymoma. About 10–15% of MG patients have thymoma (6), and the prevalence increases with age. MG is also one of the most common accessory syndromes for thymoma patients. It is estimated that between 30% and 50% of patients with thymoma or thymic carcinoma will suffer from MG, according to the 2022 National Comprehensive Cancer Network (NCCN) guidelines for thymoma and thymic carcinomas.
Currently, both thymoma and MG have corresponding international diagnostic and treatment guidelines or expert consensus, but none for thymoma patients with MG. International studies of thymoma patients with MG (7-11) mainly focus on postoperative MG, MG aggravation, thymoma recurrence, and adjuvant therapy. Chinese scholars (7) believe that MG Osserman II–IV and WHO B2-B3 thymoma are independent risk factors for MG exacerbation, while German researchers believe thymoma is an independent predictor for a myasthenic crisis (MC) or MG (12). However, when thymoma recurs, not all patients will have MG exacerbation. MG hinders patients’ performance of activities of daily living and other phenomena seriously affect their physical and mental health. Why MG worsens in some patients with recurrent thymomas but not in others, and whether it is related to the postoperative management of thymomas is unclear.
Therefore, considering the long-term prognosis of MG patients, we believed it was important to develop a predictive model for MG exacerbation in patients with postoperative recurrence of thymoma based on their clinical characteristics before the recurrence. This model can help us predict the possibility of MG exacerbation relatively accurately according to clinical factors, thus strengthening the management of high-risk groups and reducing the rate of MG exacerbation. At present, there is no relevant predictive model for reference in the available literature. Our research included both model development and validation. There are several types of predictive models, but nomograms can provide individualized, evidence-based, and accurate estimates of risk. At the same time, a nomogram is easy to use and can assist in decision making related to MG management. We present the following article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-549/rc).
Methods
Patients
Data for cases of thymoma in patients who were treated at the Center of Treatment of Myasthenia Gravis, People’s Hospital of Shijiazhuang between January 1st, 2013, and December 31th, 2021, were retrospectively collected. According to statistics, a total of 176 patients met the criteria and were included in the study. We used the patients with recurrent thymoma from 2013 to 2020 as the training cohort (n=120), and the patients in 2021 as the validation cohort (n=56). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the People’s Hospital of Shijiazhuang (No. 2022045). Informed consent was obtained from all individual participants included in the study.
Basic data of patients were collected, including age, gender, MGFA classification, WHO histological classification, pathological stage, and postoperative treatment methods to determine the possible prediction factors. Inclusion criteria were: (I) clearly diagnosed recurrent thymoma after R0 resection of thymoma; (II) thymoma combined with MG before surgery (III) no preoperative thymoma metastasis; (IV) no preoperative treatment related to thymoma or MG; (V) achieved MM-2 or better in accordance with the American Myasthenia Gravis Foundation’s Post-intervention Status (MGFA-PIS). MM-2 defined as no obvious MG symptoms, but electromyogram shows that some muscles still have weakness. At this stage, the patients can use immunosuppressants and pyridostigmine (<120 mg/day). Exclusion criteria were thymic carcinoma, concomitant with other autoimmune diseases (e.g., dermatomyositis, polymyositis, vitiligo, Sjogren syndrome, etc.), or incomplete clinical information/unknown pathology. The training cohort for creating the nomogram consisted of patients who satisfied the criteria between January 1st, 2013, and December 31st, 2020, and the validation cohort consisted of patients who fulfilled the inclusion standards between January 1st, 2021, and December 31st, 2021. All patients were followed up until March 2022. Data collation and analysis were conducted from January to April 2022, and article writing was conducted from June to July 2022.
Definition of recurrent thymoma and MG exacerbation
Preoperative diagnosis of thymoma was based on NCCN guidelines, and surgical resection included intact thymoma and surrounding tissue. The 2020 Chinese Guidelines for the diagnosis and treatment of myasthenia gravis served as the foundation for MG diagnosis. Thymoma recurrence was described as a new mass with an obvious pathologic diagnosis. Exacerbation of MG was evaluated by Xu’s absolute and relative score (ARS-MG) (13). If the difference between the recurrent and postoperative ARS-MG scores was >5 points, then MG was aggravated.
Clinical variables
The pathologic classification of thymoma was based on the World Health Organization (WHO) (14) system, whereby types A, AB, and B1 are considered low-risk tumors, and types B2 and B3 are high-risk tumors. If the tumor was of mixed pathologic type, the highest pathological classification was the criterion; for example, type B2B3 belonged to type B3 thymoma. Clinical staging was based on the Masaoka-Koga staging proposed in 1994 (14), in which stage I is considered low invasion and stages II, III, and IV are high invasion. We evaluated the severity of MG in all patients in accordance with the MGFA classification.
Treatment after tumor resection included all treatments from the end of surgery to 3 months postoperative, which included both thymoma and MG treatment, and all treatments were implemented according to the diagnostic and treatment guidelines. Special attention should be paid to the fact that high-dose steroid pulse therapy was first proposed in 2003 (15), and the MG symptoms of patients were significantly improved after its clinical application. Methylprednisolone is a commonly used drug for high-dose steroid pulse therapy; According to the scientific method, the intravenous infusion starts from 1 g/day and is reduced by half every three days until it reaches 60 mg/day. Then take methylprednisolone by mouth for 52 mg/day, and gradually reduce to discontinuation after discharge. To facilitate statistical analysis, we divided treatment plans into the 6 types shown in Table 1.
Table 1
| Treatment plan | Abbreviations |
|---|---|
| Radiotherapy + high-dose steroid pulse | RH |
| Radiotherapy + chemotherapy | RC |
| Radiotherapy + other | OR |
| High-dose steroid pulse | OH |
| Chemotherapy | OC |
| Other | OO |
RH, radiotherapy combined with high-dose steroid pulse therapy; RC, synchronous chemoradiotherapy; OR, radiotherapy combined with other treatments; OH, high-dose steroid pulse therapy only; OC, chemotherapy only; OO, other treatments.
Other treatments included low-dose steroid pulse therapy and/or cholinesterase inhibitors and/or immunosuppressants, mainly taken orally. Immunosuppressants included azathioprine, cyclophosphamide, tacrolimus, and cyclosporine, etc. The RH plan combined radiotherapy and high-dose steroid pulse therapy; RC was synchronous chemoradiotherapy; OR was radiotherapy combined with other treatments; OH comprised high-dose steroid pulse therapy only; OC was chemotherapy only; OO was other treatments.
Statistical analysis
Continuous variables were converted into classified variables, and classified variables were analyzed in proportion. To identify risk factors significantly associated with MG exacerbation, univariate analysis was conducted using the χ2 test or Fisher’s exact test, and multivariate analysis was conducted using binary logistic regression to obtain independent risk factors of MG exacerbation. Based on the outcomes of the above, we created a nomogram, which was completed using R, version 4.1, and the rms installation package. In order to better utilize the nomogram in the clinic, we quantified and assigned the risk factors that were eventually included in the predictive model. The value was calculated by the regression coefficient of each risk factor in the outcomes of multivariate analysis, with a maximum of no more than 100 points and a minimum of no less than 0 points. Every variable’s point was totaled and converted into a predicted probability. We used 1,000 bootstrap samples to calibrate the predictive model and assessed predictive efficiency with the concordance index (C-index).
We created a nomogram by using the data collected in this study and calculated each patient’s score accordingly. Receiver operating characteristic (ROC) curve analysis was used to calculate the best cut-off value according to the greatest Youden index (i.e., sensitivity + specificity – 1). The ideal cut-off value was chosen by five basic statistical indicators [sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio]. In medical statistics, any diagnosis has two basic characteristics: sensitivity and specificity. Sensitivity refers to the probability of not missing a diagnosis of a disease, while specificity refers to the probability of correctly diagnosing the disease without misdiagnosis.
All P values were two-sided, and P<0.05 was used in the analysis as the test level to determine statistical significance. The analyses were conducted with SPSS26.0 and R, version 4.1 software packages.
Results
Clinical data
During the study period, 1,018 patients with thymoma accompanied by MG were treated, and 240 with postoperative recurrent thymoma were enrolled in the study. After application of the inclusion criteria, 176 patients comprised the study group: 120 patients in the training cohort and 56 patients in the validation cohort (Figure 1). All the data required to establish the predictive model were available.
A summary of the baseline clinical characteristics of the two cohorts is given in Table 2. There was no statistical significance between them. MG exacerbation occurred in 84 (70.0%) and 41 (73.3%) patients in the two cohorts, respectively.
Table 2
| Variable | Cohort, n (%) | P value | |
|---|---|---|---|
| Training (n=120) | Validation (n=56) | ||
| Sex | 0.368 | ||
| Male | 58 (48.3) | 23 (41.1) | |
| Female | 62 (51.7) | 33 (58.9) | |
| Onset (age, years) | 0.355 | ||
| ≤50 | 103 (85.8) | 45 (80.4) | |
| >50 | 17 (14.2) | 11 (19.6) | |
| MGFA | 0.264 | ||
| I | 4 (3.3) | 0 | |
| IIa, IIb | 25 (20.8) | 10 (17.9) | |
| IIIa, IIIb | 29 (24.2) | 17 (30.4) | |
| IVa, IVb | 41 (34.2) | 14 (25.0) | |
| V | 21 (17.5) | 15 (26.7) | |
| Pathology | 0.542 | ||
| A | 4 (3.3) | 1 (1.8) | |
| AB | 13 (10.8) | 8 (14.3) | |
| B1 | 36 (30.0) | 13 (23.2) | |
| B2 | 33 (27.5) | 20 (35.7) | |
| B3 | 34 (28.3) | 14 (25.0) | |
| Masaoka-Koga stage | 0.975 | ||
| I | 41 (34.2) | 19 (33.9) | |
| II | 53 (44.2) | 22 (39.3) | |
| III | 12 (10.0) | 10 (17.9) | |
| IV | 14 (11.6) | 5 (8.9) | |
| Treatment plan | 0.082 | ||
| RH | 13 (10.8) | 5 (8.9) | |
| RC | 14 (11.7) | 7 (12.5) | |
| OR | 27 (22.5) | 11 (19.7) | |
| OH | 30 (25.0) | 5 (8.9) | |
| OC | 7 (5.8) | 7 (12.5) | |
| OO | 29 (24.2) | 21 (37.5) | |
| MG exacerbation | 0.662 | ||
| Yes | 84 (70.0) | 41 (73.3) | |
| No | 36 (30.0) | 15 (26.7) | |
MGFA, Myasthenia Gravis Foundation of America; RH, radiotherapy combined with high-dose steroid pulse therapy; RC, synchronous chemoradiotherapy; OR, radiotherapy combined with other treatments; OH, high-dose steroid pulse therapy only; OC, chemotherapy only; OO, other treatments; MG, myasthenia gravis.
Development and validation of the nomogram
The pathogenic type of thymoma (P=0.006), Masaoka-Koga stage (P=0.008), as well as postoperative treatment strategy (P=0.001) were significantly connected with MG exacerbation following thymoma recurrence, according to the findings of the univariate analysis reported in Table 3. Types A, AB, and B1 thymomas were identified as independent risk variables for MG exacerbation by multivariate analysis (Table 4) [odds ratio (OR) =3.113; 95% confidence interval (CI): 1.175–8.246]. It was discovered that OO treatment was a distinct risk factor for MG exacerbation when compared with RC and OH treatment.
Table 3
| Variable | Exacerbation (n=84) | No exacerbation (n=36) | χ² | P value |
|---|---|---|---|---|
| Sex | 3.076 | 0.079 | ||
| Male | 45 (53.6) | 13 (36.1) | ||
| Female | 39 (46.4) | 23 (63.9) | ||
| Onset (age, years) | 0.395 | 0.530 | ||
| ≤50 | 71 (84.5) | 32 (88.9) | ||
| >50 | 13 (15.5) | 4 (11.1) | ||
| MGFA | 3.733 | 0.442 | ||
| I | 4 (4.8) | 0 (0) | ||
| IIa, IIb | 16 (19.0) | 9 (25.0) | ||
| IIIa, IIIb | 18 (21.4) | 11 (30.6) | ||
| IVa, IVb | 29 (34.6) | 12 (33.3) | ||
| V | 17 (20.2) | 4 (11.1) | ||
| Pathology | 7.661 | 0.006 | ||
| A, AB, B1 | 44 (52.4) | 9 (25.0) | ||
| B2, B3 | 40 (47.6) | 27 (75.0) | ||
| Massaoka-Koga stage | 7.002 | 0.008 | ||
| I | 35 (41.7) | 6 (16.7) | ||
| II, III, IV | 49 (58.3) | 30 (83.3) | ||
| Treatment plan | 18.789 | 0.001 | ||
| RH | 8 (9.5) | 5 (13.9) | ||
| RC | 6 (7.2) | 8 (22.2) | ||
| OR | 19 (22.6) | 8 (22.2) | ||
| OH | 18 (21.4) | 12 (33.3) | ||
| OC | 5 (6.0) | 2 (5.6) | ||
| OO | 28 (33.3) | 1 (2.8) | ||
MGFA, Myasthenia Gravis Foundation of America; RH, radiotherapy combined with high-dose steroid pulse therapy; RC, synchronous chemoradiotherapy; OR, radiotherapy combined with other treatments; OH, high-dose steroid pulse therapy only; OC, chemotherapy only; OO, other treatments.
Table 4
| Variable | β | SE | OR | 95% CI | P value |
|---|---|---|---|---|---|
| Pathology (A/AB/B1 vs. B2/B3) | 1.136 | 0.497 | 3.113 | 1.175–8.246 | 0.022 |
| Masaoka-Koga stage (I vs. II/III/IV) | 1.006 | 0.644 | 2.733 | 0.773–9.666 | 0.119 |
| Treatment plan | 0.070 | ||||
| RH vs. RC | −0.556 | 0.819 | 0.5736 | 0.115–2.856 | 0.497 |
| RH vs. OR | 0.599 | 0.744 | 1.820 | 0.423–7.827 | 0.421 |
| RH vs. OH | −0.617 | 0.778 | 0.540 | 0.118–2.478 | 0.428 |
| RH vs. OC | 0.444 | 1.075 | 1.558 | 0.189–12.817 | 0.680 |
| RH vs. OO | 2.298 | 1.216 | 9.954 | 0.917–108.011 | 0.059 |
| RC vs. OR | 1.155 | 0.709 | 3.175 | 0.791–12.736 | 0.103 |
| RC vs. OH | −0.61 | 0.761 | 0.941 | 0.212–4.186 | 0.937 |
| RC vs. OC | 1.000 | 1.054 | 2.718 | 0.344–21.448 | 0.343 |
| RC vs. OO | 2.854 | 1.203 | 17.362 | 1.643–183.515 | 0.018 |
| OR vs. OH | −1.216 | 0.674 | 0.296 | 0.079–1.110 | 0.071 |
| OR vs. OC | −0.155 | 0.985 | 0.856 | 0.124–5.900 | 0.875 |
| OR vs. OO | 1.699 | 1.148 | 5.469 | 0.577–51.863 | 0.139 |
| OH vs. OC | 1.060 | 0.970 | 2.888 | 0.431–19.325 | 0.274 |
| OH vs. OO | 2.915 | 1.106 | 18.445 | 2.111–161.184 | 0.008 |
| OC vs. OO | 1.854 | 1.348 | 6.388 | 0.455–89.678 | 0.169 |
RH, radiotherapy combined with high-dose steroid pulse therapy; RC, synchronous chemoradiotherapy; OR, radiotherapy combined with other treatments; OH, high-dose steroid pulse therapy only; OC, chemotherapy only; OO, other treatments; CI, confidence interval; OR, odds ratio; SE, standard error; β, regression coefficient.
The thymoma pathology and postoperative treatment plan were included in the predictive model of MG exacerbation with postoperative thymoma recurrence (Figure 2A). One of the general techniques for assessing the discriminatory power of predictive models is the bootstrap verification approach, which we used to demonstrate the model’s correctness. The nomogram showed good accuracy in predicting the probability of MG exacerbation. The C index before adjustment was 0.77 (95% CI: 0.69–0.86), and 0.77 after internal validation. Moreover, the calibration curve was visually displayed, and the MG exacerbation risk estimated by the nomogram showed good consistency with the real-world incidence (Figure 2B).
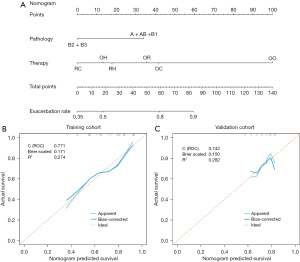
Utilizing the validation cohort data to assess the MG exacerbation predictive model, the C index was 0.74 (95% CI: 0.58–0.91). Statistically speaking, there was no difference from the training cohort’s C index. The calibration curve suitable for risk assessment is shown in Figure 2C.
Risk of MG exacerbation based on nomogram score
We regarded 60 points as the best cut-off value of the nomogram. Sensitivity, specificity, PPV and NPV were the four basic indicators to describe the predictive model and their values were 75.7% (95% CI: 66.1–83.4%), 64.7% (95% CI: 38.6–84.7%), 92.9% (95% CI: 84.5–97.1%), and 30.6% (95% CI: 16.9–48.3%) respectively in the training cohort and 84.1% (95% CI: 69.3–92.8%), 66.7% (95% CI: 35.4–88.7%), 90.2% (95% CI: 75.9–96.8%), and 53.3% (95% CI: 27.4–77.7%) in the validation cohort (Table 5).
Table 5
| Variable | Value (95% CI) | |
|---|---|---|
| Training cohort | Validation cohort | |
| AUC | 0.771 (0.686–0.855) | 0.742 (0.580–0.906) |
| Cut-off | 60 | 60 |
| Sensitivity | 0.757 (0.661–0.834) | 0.841 (0.693–0.928) |
| Specificity | 0.647 (0.386–0.847) | 0.667 (0.354–0.887) |
| Positive predictive value | 0.929 (0.845–0.971) | 0.902 (0.759–0.968) |
| Negative predictive value | 0.306 (0.169–0.483) | 0.533 (0.274–0.777) |
| Positive likelihood ratio | 2.146 (1.117–4.122) | 2.523 (1.122–5.673) |
| Negative likelihood ratio | 0.375 (0.250–0.562) | 0.239 (0.111–0.511) |
MG, myasthenia gravis; CI, confidence interval; AUC, area under the curve.
Discussion
We reviewed 1,018 thymoma patients with MG in the same hospital over 9 years (from 2013 to 2021), collected and screened their clinical data, and constructed a predictive model of MG exacerbation using the data of patients with recurrent thymoma after R0 resection. The recurrence rate of thymoma in the whole database was 23.6%, which was consistent with previous reports (2-4). Multivariate analysis showed that the WHO type of thymoma and postoperative treatment plan were independent risk factors for MG exacerbation after thymoma recurrence. At present, the recurrence rate of thymoma in the cohort suggested formulating follow-up strategies more carefully and evaluating the treatment plans.
Previous studies (16-18) have shown that the pathologic classification may be related to the recurrence of thymoma, but the relationship between the pathologic classification and the prognosis of MG was unclear. Menon et al.’s (19) study showed that the thymoma pathologic classification did not correlate with the prognosis of MG. Xue et al. (7) suggested that type B2 and type B3 thymomas were independently associated with a postoperative MC, and Kim et al. (9) reported that type A and type AB thymoma were risk factors for postoperative MG. Some results are contradictory, and we believe that differences in sample size and observation indicators may be the reason. It is worth noting that although there is no report on a MG exacerbation predictive model after thymoma recurrence, the results of Kim et al. were similar to ours. There was evidence that MG exacerbation was more likely to occur in individuals with recurrence of type A, AB, or B1 thymoma. The data revealed that 63.4% (64/101) of patients with type B2 and type B3 thymoma experienced MG exacerbation, compared with 81.3% (61/75) of those with type A, AB, and B1 thymoma. Among all the patients, type B2 thymoma accounted for the highest proportion (53/176, 30.1%), which suggests that postoperative management of patients with this pathologic classification should be strengthened.
Liu et al. (18) created a predictive model for thymoma recurrence after statistically analyzing data from the Chinese Thymoma Research Alliance (ChART) database. Based on their nomogram, patients were classified into a high-risk or low-risk group. All B2 and B3 thymomas in stage T2 or T3, as well as thymic carcinoma and neuroendocrine tumors, were classified as high risk, and all other tumor types were classified as low risk. The low-risk group had a lower rate of thymoma recurrence. However, even with the large sample size of this study, the model neglected to account for the effect of postoperative adjuvant therapy on MG exacerbation. Additionally, the TNM stage (20) was less clinically practical than the Masaoka-Koga stage. We not only included preoperative clinicopathologic data, but also postoperative adjuvant therapy. The final study results were visualized with the nomogram (21) (Figure 2A), and the training cohort’s C index was calculated to be 0.77 and 0.74 for the validation cohort. Good performance was also shown by the corresponding calibration curve.
The postoperative treatment plan was an independent predictor in our study, and OO was more likely to aggravate MG after thymoma recurrence than RC (P=0.018) or OH (P=0.008). Patients with advanced or inoperable thymoma are often treated with radiotherapy and chemotherapy, both of which are a type of immunotherapy that kills both tumor cells and lymphocytes, indirectly affecting MG symptoms. At present, several studies (10,11,22,23) have shown that postoperative radiotherapy can prolong disease-free survival (DFS) and overall survival (OS) in advanced thymoma patients. Lu et al. (24) suggested that adjuvant radiotherapy within 1 month after surgery helps control postoperative MG in thymoma patients with MG. Compared with single radiotherapy, simultaneous chemotherapy can not only kill tumors but also increase radiotherapy sensitivity (25). Steroids are a common treatment for autoimmune diseases such as MG, and can induce apoptosis of lymphocytes as a lytic agent (26). At the same time, from the perspective of cell composition (14), thymomas of different pathologic types more or less contain lymphocytes, so we believe that steroid therapy is effective for thymomas, and patients with rich lymphocyte components have a more effective response than patients with fewer. Zouvelou et al. confirmed our hypothesis by reviewing cases and finding that preoperative prednisolone therapy can cause tumor shrinkage (26).
It is particularly noteworthy that the existing international expert consensus (5,27) does not detail specific doses or maintenance time of steroids in thymoma patients with MG. We not only evaluated the steroid treatment method and dose but also confirmed for the first time the long-term benefit of high-dose steroid pulse therapy on the prognosis of MG in patients with postoperative recurrence of thymoma. On the basis of these findings, we can design prospective clinical trials or randomized controlled trials for two major purposes: determining the applicable population for high-dose steroid pulse therapy and the difference between it and low-dose steroid pulse therapy.
Concerning the clinical application of the predictive model, we assessed the sensitivity, specificity, PPV, and NPV of MG exacerbation with a critical value of 60 (Table 5). Based on the sensitivity and specificity, we believe that our nomogram has good accuracy. Patients with scores >60 points (38/176, 21.6%) were the high-risk subgroup of MG exacerbation (PPV, 91.5%). In light of this, the nomogram can be used as a tool to assess the risk of MG exacerbation after thymoma recurrence. In addition, it can also assist clinicians in postoperative adjuvant treatment selection and reduce the risk of MG exacerbation in patients.
Study limitations include single-center data, so it is important to confirm the results with data from several other institutions. Second, prospective studies are needed to further verify the nomogram’s reliability. Third, despite having strong predictive accuracy, the nomogram had a false-positive rate of 35.3% and 33.3%, and a false negative rate of 24.3% and 15.9%, respectively, in the two cohorts. When predicting MG exacerbation in patients with postoperative recurrent thymoma, these statistics are still slightly high. Finally, the long postoperative OS of thymoma patients, the low probability of recurrence after R0 resection, and the long follow-up were all reasons for the loss of follow-up bias.
Conclusions
Combining the pathologic classification of thymoma and the postoperative treatment plan, we constructed a nomogram to assess the risk of MG exacerbation with postoperative recurrence of thymoma after R0 resection.
Acknowledgments
Funding: This work was supported by financial grants from the Hebei Provincial Natural Science Foundation (H2019106063) and the S&T Program of Hebei (19277701D). The funder had no role in trial design, data collection, data analysis, data interpretation, or writing of the report.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-549/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-549/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-549/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of People’s Hospital of Shijiazhuang (No. 2022045). Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ried M, Guth H, Potzger T, et al. Surgical resection of thymoma still represents the first choice of treatment. Thorac Cardiovasc Surg 2012;60:145-9. [Crossref] [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [Crossref] [PubMed]
- Regnard JF, Zinzindohoue F, Magdeleinat P, et al. Results of re-resection for recurrent thymomas. Ann Thorac Surg 1997;64:1593-8. [Crossref] [PubMed]
- Hamaji M, Ali SO, Burt BM. A meta-analysis of surgical versus nonsurgical management of recurrent thymoma. Ann Thorac Surg 2014;98:748-55. [Crossref] [PubMed]
- Narayanaswami P, Sanders DB, Wolfe G, et al. International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology 2021;96:114-22. [Crossref] [PubMed]
- Gilhus NE. Myasthenia Gravis. N Engl J Med 2016;375:2570-81. [Crossref] [PubMed]
- Xue L, Wang L, Dong J, et al. Risk factors of myasthenic crisis after thymectomy for thymoma patients with myasthenia gravis. Eur J Cardiothorac Surg 2017;52:692-7. [Crossref] [PubMed]
- Yamada Y, Yoshida S, Iwata T, et al. Risk factors for developing postthymectomy myasthenia gravis in thymoma patients. Ann Thorac Surg 2015;99:1013-9. [Crossref] [PubMed]
- Kim A, Choi SJ, Kang CH, et al. Risk factors for developing post-thymectomy myasthenia gravis in patients with thymoma. Muscle Nerve 2021;63:531-7. [Crossref] [PubMed]
- Zhou D, Deng XF, Liu QX, et al. The Effectiveness of Postoperative Radiotherapy in Patients With Completely Resected Thymoma: A Meta-Analysis. Ann Thorac Surg 2016;101:305-10. [Crossref] [PubMed]
- Bruni A, Stefani A, Perna M, et al. The role of postoperative radiotherapy for thymomas: a multicentric retrospective evaluation from three Italian centers and review of the literature. J Thorac Dis 2020;12:7518-30. [Crossref] [PubMed]
- Nelke C, Stascheit F, Eckert C, et al. Independent risk factors for myasthenic crisis and disease exacerbation in a retrospective cohort of myasthenia gravis patients. J Neuroinflammation 2022;19:89. [Crossref] [PubMed]
- Lu B, Ye Q, Pan Y, et al. Tonifying spleen and replenishing kidney method of traditional Chinese medicine for myasthenia gravis: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e25966. [Crossref] [PubMed]
- Müller-Hermelink HK, Ströbel P, Zettl A, et al. Combined thymic epithelial tumours. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. Pathology and genetics of tumours of the lung, pleura, thymus and heart (WHO classification of tumours series). Lyon, France: IARC Press, 2004:196-8.
- Qi G, Liu P, Dong H, et al. Metastatic Thymoma-Associated Myasthenia Gravis: Favorable Response to Steroid Pulse Therapy Plus Immunosuppressive Agent. Med Sci Monit 2017;23:1217-23. [Crossref] [PubMed]
- Roden AC, Yi ES, Cassivi SD, et al. Clinicopathological features of thymic carcinomas and the impact of histopathological agreement on prognostical studies. Eur J Cardiothorac Surg 2013;43:1131-9. [Crossref] [PubMed]
- Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg 2005;80:2002-7. [Crossref] [PubMed]
- Liu H, Gu Z, Qiu B, et al. A Recurrence Predictive Model for Thymic Tumors and Its Implication for Postoperative Management: a Chinese Alliance for Research in Thymomas Database Study. J Thorac Oncol 2020;15:448-56. [Crossref] [PubMed]
- Menon D, Katzberg H, Barnett C, et al. Thymoma pathology and myasthenia gravis outcomes. Muscle Nerve 2021;63:868-73. [Crossref] [PubMed]
- Chiappetta M, Lococo F, Pogliani L, et al. Masaoka-Koga and TNM Staging System in Thymic Epithelial Tumors: Prognostic Comparison and the Role of the Number of Involved Structures. Cancers (Basel) 2021;13:5254. [Crossref] [PubMed]
- Lei Z, Li J, Wu D, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg 2016;151:356-63. [Crossref] [PubMed]
- Song SH, Suh JW, Yu WS, et al. The role of postoperative radiotherapy in stage II and III thymoma: a Korean multicenter database study. J Thorac Dis 2020;12:6680-9. [Crossref] [PubMed]
- Lim YJ, Kim E, Kim HJ, et al. Survival Impact of Adjuvant Radiation Therapy in Masaoka Stage II to IV Thymomas: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys 2016;94:1129-36. [Crossref] [PubMed]
- Lu CF, Yu L, Jing Y, et al. Value of Adjuvant Radiotherapy for Thymoma with Myasthenia Gravis after Extended Thymectomy. Chin Med J (Engl) 2018;131:927-32. [Crossref] [PubMed]
- Nagasawa S, Takahashi J, Suzuki G, et al. Why Concurrent CDDP and Radiotherapy Has Synergistic Antitumor Effects: A Review of In Vitro Experimental and Clinical-Based Studies. Int J Mol Sci 2021;22:3140. [Crossref] [PubMed]
- Zouvelou V, Vamvakaris I, Tentolouris-Piperas V, et al. The effect of glucocorticoids on radiology and histology of thymoma in myasthenia gravis. Acta Neurol Belg 2022;122:1073-5. [Crossref] [PubMed]
- Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016;87:419-25. [Crossref] [PubMed]
(English Language Editor: K. Brown)



