
The feasibility of laryngeal nerve protection during thyroidectomy using sternocleidomastoid intermuscular approach with intraoperative neuromonitoring: a case series and step-by-step description of surgical procedure
Introduction
Chinese surgeons strictly adhere to international guidelines and reserve thyroidectomy for the treatment of thyroid cancer, toxic thyroid nodules, multinodular goiter (especially when nearby structures are compressed), Graves’ disease (especially when exophthalmos is present), and thyroid nodules when the results of fine needle aspiration (FNA) are unclear (1,2). In China, more than 150,000 thyroid procedures are performed every year (3,4).
In recent years, new scientific knowledge has led to the development of less invasive procedures (endoscopy), new approaches, tailored surgery, and the application of technologies to improve the quality of dissection [i.e., intraoperative neural monitoring (IONM), energy-based devices, autoflorescence] (5).
Any new approach or technology applied to thyroidectomy requires constant review (6), because thyroidectomy in particular can result in a dysphonia complication, which is a change in the timbre of the voice due to injury to the recurrent laryngeal nerve (RLN) and subsequent impairment of vocal cord (VC) movement (7).
Monitoring of the RLN by intraoperative electromyographic (EMG) and laryngeal examinations adds to the knowledge of the appropriateness and safety of new approaches and prevention strategies (7,8).
With the continuous improvement of thyroid surgery methods, there is an increasing demand for anterior neck function and cosmetic results under the premise of ensuring safe and effective surgery. So we have adopted an alternative sternocleidomastoid intermuscular approach (SMIA) for open/conventional thyroidectomy. No matter what kind of operation, we should not only ensure the effectiveness of the operation, but also ensure the safety of the operation. Especially the protection of laryngeal nerve during operation, which is closely related to the quality of life of patients after operation. Compared with the traditional thyroid surgery, the surgical approach of SMIA has changed greatly, and the way of intraoperative nerve monitoring should be adjusted accordingly. The aim of this study was to describe SMIA and to testify the feasibility of RLN and external branch of superior laryngeal nerve (EBSLN) functional protection during SMIA thyroidectomy with the intraoperative neuromonitoring. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-500/rc).
Methods
Study design, setting, and population
The study population comprised a prospective cohort of patients undergoing thyroid surgery in a Level 3 College Hospital (Department of Thyroid Surgery, China-Japan Union Hospital of Jilin College, Changchun, China). Data were collected in a prospective database of clinical medical records maintained and quality assured by an indexing expert. We set inclusion and exclusion criteria. Included patients were recruited from a level 3 College Hospital, i.e., the Department of Thyroid Surgery, China-Japan Union Hospital of Jilin College, Changchun, China. The total duration of the study was from April 2020 to April 2022. We collected clinical and surgical data and intraoperative neurological monitoring data of these patients, and compared the EMG signal values of R1, R2, V1, V2 and EBSLN to determine whether SMIA surgery ensured the safety of RLN and EBSLN. If there is no significant difference in EMG signals between RLN and EBSLN before and after operation, and there is no abnormal laryngeal examination, it shows that SMIA surgical approach can ensure the safety of laryngeal nerve and is a safe and effective surgical method.
The Internal Review Board approved data analysis for all patients who had undergone thyroid surgery. A detailed informed consent form containing prospective data gathering, surgical protocols, and IONM procedures is signed by the patients or their legal guardians prior to surgery. Recently, the detailed IONM informed consent form was released (9). Participants’ personal information was guaranteed not to be disclosed. The study followed the Helsinki Declaration (as revised in 2013) (10) and was approved by the Institutional Review Board of China-Japan Union Hospital of Jilin University (approval number: 20220804014).
Eligibility criteria
The inclusion criteria included patients over 18 years old who underwent transcervical thyroidectomy for the first time; unilateral thyroid nodules; thyroid nodules confirmed as malignant by ultrasound-guided FNA or strongly suspected by preoperative US examination (TI-RADS 5); no significant difference in bilateral vagal EMG signal (V1 signal) (2). Exclusion criteria were a history of neck surgery; total thyroidectomy or lateral cervical lymph node dissection; preoperative VC paralysis. Surgeries with RLN monitoring but not thyroidectomy were excluded (i.e., esophageal atresia, tracheoesophageal fistula and tracheobronchomalacia, cardiac surgery). Patients with laparoscopic thyroid surgery were not included in the study. Withdrawal of informed consent by the patient or guardian was a standard for excluding from analysis. Surgeries were performed by experienced endocrine and IONM surgeons (11).
Procedures
IONM
Our study uses noninvasive monitoring systems [Nerve Integrity Monitor (NIM) Response 3.0 system, recently NIM Vital (NIM-Response 3.0, Medtronic, Jacksonville, Florida, USA)]. The IONM was used in an intermittent application mode. We intermittently stimulate the RLN and EBSLN using an aseptic, disposable, pulsant single polar stimulator probe. Stimulation parameters were set at a frequency of 30 Hz, 4 stimulations/s, impulse duration of 100 ms, and the impedance was 5 kΩ. (12). We conventionally deliver an electrical current at an intensity of 2 mA for identification of the vagus nerve (VN), RLN, and EBSLN, while 1–0.5 mA is used for confirmation of the RLN and branches (5). A nondepolarizing muscle relaxant (cis-atracurium 0.05 mg/kg or rocuronium 0.3 mg/kg) was used only during anesthetic induction. Depending on the patient’s age, body weight, and size, an appropriate EMG endotracheal tube (Medtronic NIM TriVantage EMG Tubes). VN was stimulated (V1) prior to dissection of the thyroid, whereas RLN was stimulated (R2) after initial identification (R1) and after completion of thyroidectomy. After thyroidectomy and hemostasis, the VN is stimulated again (V2) (12).
SMIA
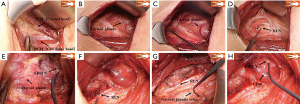
The following is a description of our alternative SMIA. Make a lateral neck arch incision ≈5 cm long through the skin, subcutaneous tissue, and platysma. Dissect between the sternal and clavicular heads of the sternocleidomastoid muscle (SCM) upward to the level of the larynx (Figure 1A), protecting the vessels and nerve tissue in the cervical sheath. The inner border of the internal jugular vein is exposed on the affected side. The posterior lateral aspect of the thyroid gland is exposed anterior to the cervical sheath between the sternal and clavicular heads (Figure 1B). Bilateral VN EMG signals are monitored and recorded as V1 (Figure 1C). The location and size of the tumor are assessed. The “cross” method is used to locate and protect the bilateral cervical RLNs, and the signal value R1 is recorded (Figure 1D). The blood vessels of the upper thyroid pole are processed to locate and protect the EBSLN (Figure 1E). The middle thyroid vein and inferior thyroid arteries and veins are transected, and extracapsular surgery performed to accurately protect the parathyroid glands and RLN. The cone-shaped lobe is separated inward along with the cricothyroid space, and the isthmus of the thyroid lobe is cut through on the healthy side. The thyroid gland and its internal tumor are removed on the affected side. The EMG signals of the RLNs are monitored in real time during resection, and the signal changes compared. When the RLN is exposed up to the Berry ligament, the nearest end of the exposed portion is tested and recorded as EMG signal R2 (Figure 1F). Finally, the lymph nodes in the central part of the affected side were excised. The excised tissue is sent for rapid pathological examination. After complete hemostasis, the bilateral EMG signal V2 is obtained and recorded (Figure 1G). Monitoring EBSLN again and observing CTM twitch (Figure 1H). After checking the gauze swabs and instruments, the wound is washed with warm saline and a drainage tube placed in the wound cavity. The incision is closed layer by layer and bandaged with pressure.
Primary targets
To evaluate the incidence of transient or deterministic injury of recurrent laryngeal nerve. The incidence of recurrent laryngeal nerve paralysis in at-risk nerves (NAR) was calculated. An independent laryngologists used pediatric laryngoscope to complete preoperative and postoperative follow-up within 24–48 hours before and after operation (7). Any limitation or abnormality in VC movement is defined as postoperative VC paralysis. Laryngoscope is reexamined 3 and 6 months after operation until it is confirmed that the vocal cord movement function has fully recovered (7).
Secondary outcomes
The characteristics and results of IONM-related EMG profiles for both the RLNs and the EBSLN. EMG profiles have been described in detail previously (6,12,13).
Statistical analysis
All the statistical analysis is carried out through SPSS 23.0 software (IBM, Armonk, NY, USA). The measured data of normal distribution are expressed by mean ± standard deviation. Student’s t-test was used to compare the differences between groups. All tests were bilateral, and the P value <0.05 was considered statistically significant.
Results
Demographic data
A total of 39 patients were enrolled in the study [5 men, 34 women; mean age 34.1±8.7 years; mean body mass index 22.5 (±3.0, 17.0–30.8) kg/m2]. Complete follow-up was available for all patients.
Interventions
All patients underwent unilateral thyroidectomy, isthmus resection, and prophylactic central lymph node dissection. The mean duration of surgery was 88.5 (±21.2, 60–140) min. The parathyroid gland was re-implanted in 1 (2.5%) patient.
Pathology
There were 37 cases (94.9%) of tumor malignancy with 15 (38.5%) lymph node metastases. The mean nodal size was 8.9 (±6.9, 3.6–36.5) mm.
Morbidity
There were no deaths or cervical hematomas requiring reoperation. None of the patients experienced transient hypocalcemia and none had wound seromas.
IONM
NAR
The number of NAR was 39:17 on the left and 22 on the right. All RLNs and EBSLN were successfully monitored.
RLN EMG profiles
In general, we analyzed the IONM data for the RLN and showed that preoperative amplitude values of 1,211 (±382, 500–1,674) µV on the right side and 1,277 (±501, 570–1,930) µV on the left side and postoperatively 1,375 (±778, 534–2,812) µV on the right side and 1,068 (±508, 415–1,851) µV on the left side.
EBSLN EMG profiles
In general, we analyzed the IONM data for the EBSLN and showed that preoperative amplitude values of 234 (±69, 112–356) µV on the right side and 256 (±100, 96–432) µV on the left side and postoperatively 219 (±56, 115–287) µV on the right and 234 (±86, 91–376) µV on the left side.
RLN injury
L2 were normal in all patients. We also compared V2 and V1 (1,236±672 vs. 1,240±428, P=0.973), R2 and R1 (1,676±778 vs. 1,656±765, P=0.849) signals separately, and the results were not statistically different (P>0.05) (Table 1). At the same time, we compared the V1 (1,240±428 vs. 1,309±395, P=0.601) signals of both RLNs, and the results showed no statistical difference (P>0.05) (Table 2).
Table 1
| IONM signal | EMG amplitude (μV) | P value |
|---|---|---|
| V | 0.973 | |
| V1 | 1,240±428 | |
| V2 | 1,236±672 | |
| R | 0.849 | |
| R1 | 1,656±765 | |
| R2 | 1,676±778 |
EMG data are expressed as mean ± standard deviation. V, vagus nerve; R, recurrent laryngeal nerve; V1, EMG amplitude of the vagus nerve; V2, EMG amplitude of vagus nerve after thyroidectomy; R1, EMG amplitude at initial identification of recurrent laryngeal nerve; R2, EMG amplitude of stimulated recurrent laryngeal nerve after completion of recurrent laryngeal nerve dissection. EMG, electromyographic; IONM, intraoperative neuromonitoring; SMIA, sternocleidomastoid intermuscular approach.
Table 2
| IONM signal | EMG amplitude (μV) | P value |
|---|---|---|
| V1 | 0.601 | |
| Affected side | 1,240±428 | |
| Healthy side | 1,309±395 |
EMG data are expressed as mean ± standard deviation. V1, EMG amplitude of the vagus nerve before thyroidectomy. EMG, electromyographic; IONM, intraoperative neuromonitoring.
EBSLN injury
CTM twitch and EMG were detected in all patients before (S1) and after (S2) intraoperative dissection of the superior thyroid vessels (13).
Discussion
Compared with traditional linea alba cervicalis approach (LACA) thyroid surgery, the anatomical pathway to the thyroid gland has changed greatly in the SMIA and therefore the use of IONM during surgery must also be adjusted.
Protection of the RLN is essential in thyroid surgery and IONM can more accurately and sensitively assess RLN function. The most common cause of RLN injury in thyroid surgery is traction of the nerve during surgery (14). During thyroid surgery using the SMIA, the thyroid gland is pulled upward to widen the surgical space in the area where the RLN passes. The surgeon performs the surgical procedure from the side and back of the thyroid gland. Compared to the top-down anatomical approach of traditional surgery, the SMIA allows the RLN to be exposed anatomically at the same level and in accordance with the anatomical positions. Complemented by real-time IONM monitoring, this reduces the likelihood of traction damage to the RLN caused by the surrounding tissue being at different anatomical levels. At the same time, the visual field of SMIA is clearer and more intuitive. Combined with IONM, we can more accurately find and distinguish the course of the RLN and expose it completely and safely. The tighter connective tissue of the Berry ligament makes dissection more difficult and can damage the nerve if careless. The use of IONM to monitor the signal of the RLN in real time and observe changes in the signal value during surgery can increase the safety of surgical operations in the high-risk area of the RLN and improve the thoroughness of thyroid lobectomy. Intraoperative EMG signal analysis in this study showed no significant difference between V2 and V1 or R2 and R1. Postoperative laryngoscopic results were normal in all patients, and there were no symptoms of VC paralysis or hoarseness. Compared with the RLN damage rate of traditional surgical methods reported in the literature, the SMIA surgery caused no RLN damage and there was more accurate and precise RLN protection due to the unique surgical pathway, clear surgical field of view, clear anatomical relationship, and real-time quantitative index feedback of IONM.
Protection of the RLN has always been the focus of thyroid surgery, but the importance of the EBSLN is often underestimated by surgeons. With the increasing demands for sound quality and patient life satisfaction after thyroid surgery, as well as the popularity of IONM, more and more attention has been paid to the detection and functional protection of the ESLN. In thyroid surgery, treatment of the upper pole of the thyroid gland is an important step in thyroid lobectomy. In the treatment of the upper pole of thyroid by LACA thyroid surgery, the thyroid gland is pulled down by releasing the cricothyroid space, exposing the blood vessels of the upper pole of thyroid, and gradually ligating or closing the gland. Three classifications have been proposed based on the location of EBSLN in relation to the suprathyroid vessels and the superior thyroid margin (15,16). Types 2A and 2B are closely associated with the superior thyroid vessels and injury occurs easily during intraoperative dissection or ligation of the superior thyroid vessels. During the actual surgery, surgeons often encounter the situation in which the upper border of the thyroid gland is elevated due to a thyroid tumor at the upper pole of the gland, a short neck, or various other reasons. Without cutting the thoracic thyroid muscle, the surgical field of the upper pole of the thyroid gland cannot be fully exposed, which makes it difficult to anatomically identify and functionally protect the EBSLN (17). Although some believe that experienced surgeons benefit less from IONM and that simple avoidance of the EBSLN is equally effective in protecting nerve function, detection of the EBSLN may be useful, especially in the above cases. The reported visual detection rate of EBSLN during thyroidectomy is 21.2–98.3%, but has significantly increased with the help of IONM (18). By separating the space of the SCM branches (between the sternal and clavicular heads), SMIA thyroidectomy pulls the sternal thyroid muscle upward and medially away from the surface of the gland, allowing the extreme surgical field to be fully exposed without cutting off the sternal thyroid muscle and increasing collateral damage. By identifying the vessels and branches of the upper pole under direct vision and guidance of IONM, the detection rate of EBSLN is further improved, which protects the EBSLN from the high risk of injury. Because EBSLN is closely related to the upper pole of thyroid gland, a smaller stimulation current should be used during surgery to stimulate the conduction pathway area. Real-time observation of CTM twitch can identify the upper thyroid vessels and nerves, determine the EBSLN classification, and safely treat the upper thyroid. In this study, CTM twitch was observed in all patients when the EBSLN was stimulated after treating the upper pole of the thyroid gland with a neural probe. Using IONM to monitor the EBSLN in SMIA can better protect the functions of the EBSLN and shorten the operation time under the safe and standardized operation.
In addition to protecting the RLN and EBSLN, the use of IONM during SMIA surgery allows effective identification and protection of all motor nerves in the surgical area, which is beneficial for functional protection of the anterior neck region during thyroid surgery. The strap muscle is innervated by two branches, of which the superior and inferior branches of the sternothyroid muscle are usually penetrated by the lateral edge or deep surface of the muscle. Surgery using SMIA minimizes damage to these nerves, and IONM allows timely detection and identification of nerve alignment. Compared with LACA, SMIA for thyroid surgery does not require a free flap, minimizing adhesions at all levels in the anterior cervical region and resulting in better surgical outcome. The postoperative incision scar is smaller, which can effectively resolve the various degrees of swallowing pulling sensation, anterior cervical foreign body sensation, and pressure sensation that exist after LACA. The postoperative quality of life is improved when the cosmetic results of neck surgery are guaranteed.
To maximize the exposure of the lateral incision plane and facilitate the surgical procedure, the patient’s head is restrained during surgery using an elastic band, and slightly tilted toward the healthy side to fully expose the incision plane face up. The IONM not only monitors the nerve on the affected side, but also the nerve signal on the healthy side to avoid accidental injury of the healthy RLN during lymph node dissection in the affected central region. In this study, the V1 signal of the contralateral RLN was measured in all patients, and there was no statistical difference compared with the signal value of the affected side (P>0.05) (Table 2). Because it is impossible to distinguish the RLN and VN based on their anatomical positions when monitoring the signals of the contralateral nerves, we can distinguish them based on the latency of the signal value of nerve monitoring (18-21). The latency of the ipsilateral VN is slightly longer than that of the RLN, so the monitored nerve can be better identified intuitively. Due to the particular head position of the patient, the V1 signal value may be too low during nerve monitoring in surgery using SMIA, which can be solved as follows. (I) Adjust the monitoring catheter. If the depth of the monitoring catheter changes or the catheter is deflected during patient position adjustment, a “false waveform” or false positive stimulus signal will occur. Work with the anesthetists to properly adjust and fix the position of the monitoring catheter to ensure smooth nerve monitoring. (II) Replace the connection of the signal channel of the interface box. The potential difference between the electrodes on both sides of the tracheal tube can be changed by the joint of the cross interface box. Based on this physical principle, the common problem of a weak V1 signal during surgery can be effectively solved. For other failures of nerve monitoring during surgery, please refer to the root cause analysis and solutions for common failures of IONM in the manufacturer’s guide.
This study had some limitations. The small sample size and data from a single center may bias the results to some degree. It is expected that a larger sample size and multicenter data will verify the results of this study. Second, the patients included in the study were exclusively those with thyroid cancer with small tumor diameter, and there were no cases in which SMIA surgery was used in the operation of larger benign thyroid nodules. Thus, it is not possible to discuss the protection of the laryngeal nerve in such cases.
Acknowledgments
Funding: This work was supported by the Science and Technology Program of Jilin Provincial Finance Department (Nos. 2020SCZ03; 2019SCZ029) and Jilin Provincial Education Department (No. JJKH20221065KJ).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-500/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-500/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-500/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of China-Japan Union Hospital of Jilin University (approval number: 20220804014). A detailed informed consent form containing prospective data gathering, surgical protocols, and IONM procedures is signed by the patients or their legal guardians prior to surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gao M, Ge M, Ji Q, et al. 2016 Chinese expert consensus and guidelines for the diagnosis and treatment of papillary thyroid microcarcinoma. Cancer Biol Med 2017;14:203-11. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Sui C, Liang N, Du R, et al. Time trend analysis of thyroid cancer surgery in China: single institutional database analysis of 15,000 patients. Endocrine 2020;68:617-28. [Crossref] [PubMed]
- Guidelines for diagnosis and treatment of thyroid nodules and differentiated thyroid cancer. Chin J Clin Oncol 2012;39:1249-72.
- Sun H, Tian W, Jiang K, et al. Clinical guidelines on intraoperative neuromonitoring during thyroid and parathyroid surgery. Ann Transl Med 2015;3:213. [PubMed]
- Sun H, Dionigi G. Additions of neural monitoring for thyroid surgery. Endocrine 2018;61:547. [Crossref] [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Postoperative laryngoscopy in thyroid surgery: proper timing to detect recurrent laryngeal nerve injury. Langenbecks Arch Surg 2010;395:327-31. [Crossref] [PubMed]
- Zhang D, Sun H, Tufano R, et al. Recurrent laryngeal nerve management in transoral endoscopic thyroidectomy. Oral Oncol 2020;108:104755. [Crossref] [PubMed]
- Wu CW, Huang TY, Randolph GW, et al. Informed Consent for Intraoperative Neural Monitoring in Thyroid and Parathyroid Surgery - Consensus Statement of the International Neural Monitoring Study Group. Front Endocrinol (Lausanne) 2021;12:795281. [Crossref] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Wu CW, Randolph GW, Barczyński M, et al. Training Courses in Laryngeal Nerve Monitoring in Thyroid and Parathyroid Surgery- The INMSG Consensus Statement. Front Endocrinol (Lausanne) 2021;12:705346. [Crossref] [PubMed]
- Randolph GW, Dralle HInternational Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Barczyński M, Randolph GW, Cernea CR, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013;123:S1-14. [Crossref] [PubMed]
- Snyder SK, Sigmond BR, Lairmore TC, et al. The long-term impact of routine intraoperative nerve monitoring during thyroid and parathyroid surgery. Surgery 2013;154:704-11; discussion 711-3. [Crossref] [PubMed]
- Cernea CR, Ferraz AR, Nishio S, et al. Surgical anatomy of the external branch of the superior laryngeal nerve. Head Neck 1992;14:380-3. [Crossref] [PubMed]
- Bellantone R, Boscherini M, Lombardi CP, et al. Is the identification of the external branch of the superior laryngeal nerve mandatory in thyroid operation? Results of a prospective randomized study. Surgery 2001;130:1055-9. [Crossref] [PubMed]
- Kierner AC, Aigner M, Burian M. The external branch of the superior laryngeal nerve: its topographical anatomy as related to surgery of the neck. Arch Otolaryngol Head Neck Surg 1998;124:301-3. [Crossref] [PubMed]
- Choi HW, Ji YB, Kim E, et al. Success rate and learning curve of intraoperative neural monitoring of the external branch of the superior laryngeal nerve in thyroidectomy. Head Neck 2021;43:3946-54. [Crossref] [PubMed]
- Zhao Y, Li C, Zhang D, et al. Experimental study of needle recording electrodes placed on the thyroid cartilage for neuromonitoring during thyroid surgery. Br J Surg 2019;106:245-54. [Crossref] [PubMed]
- Zhao Y, Zhao Z, Zhang D, et al. Improving classification of the external branch of the superior laryngeal nerve with neural monitoring: a research appraisal and narrative review. Gland Surg 2021;10:2847-60. [Crossref] [PubMed]
- Zhao Y, Zhao Z, Wang T, et al. The area under the waveform of electromyography for monitoring the external branches of the superior laryngeal nerve during thyroid surgery. Gland Surg 2021;10:143-53. [Crossref] [PubMed]
(English Language Editor: K. Brown)


