
Lip pleomorphic adenomas: case series and literature review
Introduction
Minor salivary glands are independent tissues of salivary cells and are present in the mucosa of the oral cavity and throat (1,2). Unlike the major salivary glands, the minor salivary glands secrete saliva directly from the salivary gland tissue into the oral cavity without passing through a duct. Minor salivary glands are not well visualized on computed tomography (CT) or magnetic resonance (MR) imaging, but in some cases, they can be the source of neoplastic lesions (3). Most of these lesions are malignant, but benign tumors occasionally develop. The hard and soft palates are the most common sites of minor salivary gland tumors, whereas the lip is a relatively rare site.
Pleomorphic adenoma (PA) is the most frequent benign salivary gland neoplasm, occurring most frequently in the parotid gland among the major salivary glands and in the palatine gland among the minor salivary glands (4,5). The occurrence of a PA in a lip is rare. This tumor is described as pleomorphic or mixed because it shows a variety of histopathologies and is composed of a mixture of glandular epithelium, myoepithelial cells, and connective tissue elements (5). PAs appear as asymptomatic firm masses with a slow growth rate, and they tend to be left untreated for a relatively long time before medical attention is sought.
In this article, we describe three rare cases of PA occurring in the lip treated at our department, and we present the results of our analysis of the relevant previous cases with a literature review. We present the following article in accordance with the AME Case Series reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-308/rc).
Methods
Study participants
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and Okayama University Hospital (approval No. 1804-015). The specimens examined in this study had been used for pathological diagnosis for treatment determination. No extra tissue was collected for this study. We obtained comprehensive informed consent from the patients to use the post-diagnosis specimens in this study.
Patients were included when they had: (I) a PA confirmed by a pathological examination, (II) high-quality images, and (III) complete clinical data. Patients without pathological results and patients whose images were poor-quality were excluded. Based on these criteria, we identified and report herein the cases of three of the total of six patients with a lip PA who visited our Department of Oral and Maxillofacial Surgery, Okayama University Hospital (Okayama, Japan) during the 20-year period from 2001 to 2021. We also analyzed the PAs in the oral region and lesions on the lips treated at our department during the same 20-year period and compared them with the existing literature regarding lip PAs.
Results
Table 1 summarizes the detailed characteristics of the six patients with a lip PA treated at our department. The average age of the patients (three males, three females) was 49.3±16.6 years (range, 33–69 years). The upper to lower lip ratio of the lesion location was 5:1, revealing a predominance of upper-lip PAs (upper: Patients 1, 2, and 4–6; lower: Patient 3). The average size of the tumors was 1.5±0.7 cm (range, 0.7–2.2 cm). The average disease period of PA was 4.9 years (range, 0.5–10 years). As an imaging method for diagnostic assistance, ultrasonography (US) was used in four cases (Patients 2–5), MR was used in three cases (Patients 2, 3, and 5), and none was used in two cases (Patients 1 and 6). As differential clinical diagnoses, benign tumor and minor salivary gland tumor were candidates. We next describe three typical cases.
Table 1
| Pt. no. | Age, years | Sex | Site | Size, cm | Disease period* | Imaging method | Clinical diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 35 | F | U | 0.7 | 7 yrs | None | Benign tumor |
| 2 | 68 | M | U | 1.3×1.3 | 10 yrs | US, MR | Benign tumor |
| 3 | 69 | M | L | 1.5×2.0 | 3 yrs | US, MR | Minor salivary gland tumor |
| 4 | 54 | F | U | 0.8 | 4 yrs | US | Minor salivary gland tumor |
| 5 | 37 | M | U | 2.2×1.2 | 6 mo | US, MR | Minor salivary gland tumor |
| 6 | 33 | F | U | 2.0 | – | None | Benign tumor |
*, from the time of self-awareness or suggestion by others to the time of the first visit; –, unclear. PA, pleiomorphic adenoma; Pt, patient; F, female; U, upper; yrs, years; M, male; US, ultrasonography; MR, magnetic resonance; L, lower; mo, months.
Patient 1
A 35-year-old Japanese woman was referred to our department for the examination of a mass on her upper lip. At her first visit, the extraoral evaluation revealed a slight swelling change in her right upper lip (Figure 1A). She had been aware of the mass for 7 years and had taken no action because it was painless. A palpation examination showed a spherical mass measuring 7 mm in diameter with a smooth surface inside the right upper lip (Figure 1B). The mass was rigid, mobile, and not tender. The patient had no significant medical history or medication. We suspected a benign tumor (e.g., an irritation fibroma) and performed an excisional biopsy to establish a definite diagnosis.
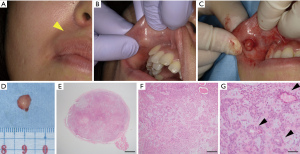
Tumor resection was performed under local anesthesia (Figure 1C). The tumor in the upper lip was surrounded by a capsule, and the tumor was removed as a single mass including the capsule (Figure 1D). The histopathological examination revealed many cells with round nuclei and eosinophilic cytoplasm inside the tumor by hematoxylin and eosin (H&E) staining (Figure 1E-1G). The resected tumor was encapsulated by a thin fibrous coating (Figure 1E). The tumor was composed of variable epithelial and myoepithelial/stromal components in a mixture of patterns (Figure 1F,1G). The epithelial cells formed ductal structures, and myoepithelial cells were observed surrounding the outside of the duct (Figure 1G). Based on these histopathological findings, we finally diagnosed the PA in the upper lip. We have been conducting a regular follow-up for 6 months, and there has been no sign of local recurrence.
Patient 2
A 68-year-old Japanese man was referred to our department with an enlarged mass on his upper lip. He had been aware of the mass for ~10 years and had taken no action because it was painless. At his first visit, the physical examination showed a movable mass measuring approximately 10 mm in diameter with a smooth surface inside the left upper lip. The mass was rigid and not tender. The patient had no significant medical history.
MR imaging revealed mid-level signal intensity in the left upper lip on the T1-weighted image (Figure 2A) and low-to-high signal intensity on the short T1 inversion recovery (STIR) image and fat-suppressed T1-weighted image (Figure 2B). The border between the tumor and the surrounding area was almost clear, but partially uneven. US revealed a well-defined circular area with no internal blood flow (Figure 2C). Based on these results, we suspected a clinically benign salivary gland tumor and planned an excisional biopsy to establish a definite diagnosis at the patient’s request.
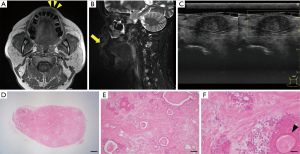
Tumor resection was performed under local anesthesia. The histopathological examination of the resected tumor demonstrated that the tumor was not encapsulated completely by a thin fibrous tissue (Figure 2D). The tumor was composed of variable epithelial and myoepithelial/stromal components in a mixture of patterns. There were ductal structures inside the tumor and myoepithelial component proliferations around the structures. Spindled myoepithelial cells streamed from ductal elements into the fibrous stroma. Some of the epithelial cells had squamous metaplasia (Figure 2E,2F). Based on these histopathological findings, we diagnosed a PA in the upper lip. There has been no sign of local recurrence to date, 3 years after the tumor’s resection.
Patient 3
A 69-year-old Japanese man was referred to our department with an enlarged mass on his lower lip. He had been aware of the mass for ~3 years, and had taken no action because he felt that it was small and inconsequential. At the patient’s first visit, the physical examination showed a movable mass measuring approx.; 20 mm in diameter with a smooth surface inside the left lower lip (Figure 3A). The patient had hepatitis B virus and was being treated for it.
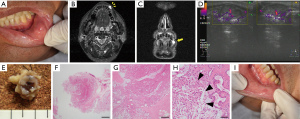
MR imaging revealed uneven signal intensity on the T1-weighted image and high signal intensity on the STIR image in the left lower lip (Figure 3B,3C). US revealed that the border between the tumor and the surrounding area was clear, and part of the border was lobular-shaped (Figure 3D). We suspected a minor salivary gland tumor and performed an excisional biopsy to establish the definite diagnosis.
Tumor resection was performed under local anesthesia. The resected tumor in the lower lip was resected as a single mass, but its morphology was irregular (Figure 3E). The histopathological examination of the resected tumor revealed that the tumor was surrounded by a thin fibrous coating, but the surgical margin was unclear (Figure 3F). The tumor was composed of variable epithelial and myoepithelial/stromal components, and plasmacytoid structures were also observed (Figure 3G,3H). Based on these histopathological findings, we diagnosed a lower-lip PA. At the time of follow-up 2 weeks after the excisional biopsy, the healing process of the wound was good (Figure 3I). There has been no sign of local recurrence to date.
PAs and lip lesions experienced in our department
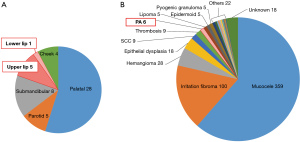
Over the past 20 years, our department has experienced a total of 51 cases of PA, of which six (11.8%) were on the lips (Figure 4). Of the six cases of lip PAs, five (9.8%) were in the upper lip and the other (2.0%) was in the lower lip (Table 1, Figure 4). Our department has encountered a total of 590 cases of mass lesions of the lips in the past 20 years, of which only approx.; 1% were resected as PAs (Figure 4).
The present investigation is limited by two factors: (I) the patient series included only individuals who underwent oral surgery (otolaryngology and plastic surgery patients were not included), and (II) the target disease was only resected lesions (untreated lesions and lesions that were treated only medically were excluded). The patient series being drawn from a single institution also necessitates further research involving a larger number of patients.
Discussion
Among all salivary gland tumors, PA is the most common, occurring in 60–70% of cases, with 84% in the parotid gland, 8% in the submandibular gland, 0.5% in the sublingual line, 6.5% in minor salivary glands, and 1% in others (6). PA of the minor salivary glands occurs in the palate (67.5%), lips (10%), cheeks (5%), tongue (2.5%), and other sites (15%); PA in a lip is relatively rare (7).
Although the cases of lip PA at our department accounted for a relatively large proportion compared to the above reports, it should be noted that many cases of PA in a parotid gland were referred to otolaryngology (only five cases of PAs of the parotid gland were treated at our department). In a comparison of the frequency of PA occurrence in the upper and lower lips, Bernier reported that of 38 cases, 35 (92.1%) were in the upper lip and three (7.9%) were in the lower lip (8). Krolls and Hicks described that of 4,042 cases of PA, 445 originated from minor salivary glands, of which 16.9% were located in the upper lip and 2.9% in the lower lip (9). The peak incidence of PA in lips was in the third and fourth decades of life, with most of the cases occurring on the upper lip (8-13).
We conducted a search of the literature on lip PAs (in Japan and worldwide) and found 35 cases described in 31 reports since 2000 (Table 2) (10,11,14-17,19-22,26-39,41). Of the 35 past cases of lip PA, 30 (85.7%) were in the upper lip (10,11,13,15,17,19-21,23-26,28-30,32-41) and only five (14.3%) were in the lower lip (14,16,22,27,31). The male-to-female ratio of the lip PAs was 4:3 (a nonsignificant difference), and the age range was from 10 to 72 years (Table 2).
Table 2
| No. | Authors | Year | Age | Sex | Site | Size, cm | Disease period* | Imaging method | Clinical diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | To et al., (14) | 2002 | 25 | M | L | 1.0 | 3 yrs | None | Benign minor salivary gland tumor |
| 2 | Jorge et al., (15) | 2002 | 15 | F | U | 1.0 | 2 mo | None | Benign mesenchymal neoplasm, neuroma, neurofibroma |
| 3 | 18 | F | U | 1.0 | 1 yr | None | Benign mesenchymal neoplasm, neuroma, neurofibroma | ||
| 4 | Lotufo et al., (10) | 2008 | 12 | M | U | 2.0 | 1 yr | None | Benign minor salivary gland tumor, lipoma |
| 5 | Moritani et al., (16) | 2008 | 72 | F | U | 1.1×1.5×1.5 | 1 yr | US | Benign tumor |
| 6 | 60 | M | L | 1.3 | 10 mo | US, MR | Benign tumor | ||
| 7 | Asuquo et al., (17) | 2009 | 50 | F | U | 16 | 2 yrs | None | – |
| 8 | 40 | F | U | 4.0×3.0 | 10 yrs | None | – | ||
| 9 | McNamara et al., (18) | 2009 | 55 | F | U | 1.0 | 30 yrs | None | – |
| 10 | Debnath & Adhyapok, (19) | 2010 | 55 | F | U | 1.5–2.0 | 1 yr | None | Benign minor salivary gland tumor, lipoma |
| 11 | Shrestha et al., (20) | 2010 | 27 | F | U | 4.5–5.0 | 3 yrs | None | Benign minor salivary gland tumor |
| 12 | Ali et al., (21) | 2011 | 33 | M | U | 3.0 | 1 yr, 6–7 mo | CT | Granuloma, benign minor salivary gland tumor |
| 13 | Kataria et al., (11) | 2011 | 65 | F | U | 2.0×1.5 | 2 yrs | None | PA |
| 14 | Sengul et al., (22) | 2011 | 49 | M | L | 1.5×0.7 | – | None | – |
| 15 | Dyalram et al., (23) | 2012 | 72 | M | U | 2.2×2.4 | 5 yrs | MR | PA |
| 16 | Mitate et al., (24) | 2013 | 55 | M | U | “little finger” | 8 yrs | MR | PA |
| 17 | Mariano et al., (25) | 2013 | 69 | M | U | 2.0×2.0 | 4 yrs | None | PA, canalicular adenoma |
| 18 | Tzermpos et al., (26) | 2014 | 39 | F | U | 1.0×0.7 | 3 yrs | None | Periapical granuloma, periapical cyst |
| 19 | Sood et al., (27) | 2014 | 46 | M | L | 1.5×1.2 | 2 yrs | None | Lipoma, sebaceous cyst |
| 20 | Fomete et al., (28) | 2015 | 37 | F | U | 4.0×3.0×2.0 | 4 yrs | None | – |
| 21 | Singh et al., (29) | 2015 | 55 | M | U | 2.0×1.5 | 1 yr | None | Benign minor salivary gland tumor, mesenchymal tumor |
| 22 | Khan et al., (30) | 2016 | 60 | M | U | 3.0×4.0 | 8 yrs | None | – |
| 23 | Taniguchi et al., (31) | 2016 | 72 | F | L | 1.0×1.5 | 7 yrs | MR | Benign tumor |
| 24 | Metgud et al., (32) | 2016 | 30 | M | U | 1.0×1.0 | 5–6 mo | None | Lipoma, sebaceous cyst |
| 25 | Fatahzadeh et al., (33) | 2017 | 58 | M | U | 1.5 | “many yrs” | – | – |
| 26 | Ahmedi et al., (34) | 2017 | 10 | F | U | 2.0×1.5 | 3 yrs | None | Lipoma |
| 27 | Alves et al., (35) | 2018 | 18 | M | U | 3.0 | 1 yr | None | – |
| 28 | Taiwo et al., (36) | 2018 | 33 | M | U | 4.0 | 3 yrs | None | Lipoma |
| 29 | Yoshimura et al., (37) | 2018 | 52 | M | U | 1.5 | 1 yr | CT, US | Benign tumor |
| 30 | Bhatia, (13) | 2019 | 23 | F | U | 1.6×1.8 | 3 mo | CT | PA |
| 31 | Nourwali & Dar-odeh, (38) | 2019 | 26 | M | U | 2.3×2.2×1.8 | 2 yrs | US | Mesenchymal tumor (fibroma), PA, sialolithiasis |
| 32 | Kazikdas et al., (39) | 2020 | 20 | M | U | 4.0×3.0 | 2 yrs | CT | Benign mixed salivary gland tumor |
| 33 | Shome et al., (40) | 2020 | 25 | F | U | 1.5×2.0 | 2 yrs | None | Benign salivary gland neoplasm |
| 34 | Adiyodi et al., (41) | 2020 | 44 | M | U | 3.0×3.0 | 5 yrs | None | Peripheral giant cell granuloma, minor salivary gland tumor, lipoma |
| 35 | 44 | M | U | 4.0×3.0 | 5 yrs | None | Peripheral giant-cell granuloma, minor salivary gland tumor, lipoma | ||
| 36 | Umemori et al. (present) | 2022 | 35 | F | U | 0.7 | 7 yrs | None | Benign tumor |
| 37 | 68 | M | U | 1.3×1.3 | 10 yrs | US, MR | Benign tumor | ||
| 38 | 69 | M | L | 15×20 | 3 yrs | US, MR | Minor salivary gland tumor |
*, from the time of self-awareness or suggestion by others to the time of the first visit; –, unclear. PA, pleiomorphic adenoma; M, male; L, lower; yr(s), year(s); F, female; U, upper; mo, months; US, ultrasonography; MR, magnetic resonance; CT, computed tomography.
Krolls and Hicks suggested that the difference in the frequency of PA between the upper and lower lips is due to the fact that, from an embryological point of view, the upper lip is formed by the fusion of three protuberances, whereas the lower lip is formed by the fusion of two protuberances, and thus embryonic cells are more likely to stray into the upper lip than the lower lip (9). Another potential reason is the difference in the number and distribution of labial glands between the upper and lower lips (3,42). Specifically, the upper labial glands are densely located between and scattered outside the corners of the mouth, while the lower labial glands are scattered between and densely located outside the corners of the mouth (3,42). The upper lip thus has a large number of well-developed labial glands, whereas the lower lip has only a few small labial glands, which may also contribute to the difference in the frequency of upper- versus lower-lip PAs (3,42).
PAs, especially of the lips, tend to be left untreated for a relatively long period of time before medical attention is sought, because they develop slowly and are rarely accompanied by ulceration or pain (8). One of our patients had been aware of the mass for ~10 years prior to the first visit, but it had been left untreated (Figure 1). One of the reasons why PAs of the lips are left untreated is that they tend to be smaller than those at other sites (43). For example, the PA of Patient 1 was 5×7 mm in dia., which is smaller than the reported PAs (10–160 mm in dia.; mean, 26 mm) (Figure 1, Table 2).
The clinical differential diagnosis of swelling of the lip includes cystic diseases (e.g., mucocele, dermoid cyst, epidermoid cyst), benign tumors (e.g., fibroma, hemangioma, lipoma, schwannoma), and malignant tumors [e.g., squamous cell carcinoma (SCC), mucoepidermoid carcinoma, adenoid cystic carcinoma], as well as foreign body stray, infection, orofacial granulomatosis, Quincke’s edema, tuberculosis, and actinomycosis (13,26,29).
For the diagnosis of soft tissue lesions in the oral cavity, MRI and US are effective for confirming the location, size, nature, and morphology of the tumor (44,45). A preoperative diagnosis is particularly important for primary salivary gland tumors of the labial gland, as malignancy is observed in 20–30% of cases (46,47). However, in many cases, it is difficult to preoperatively diagnose minor salivary gland tumors, especially in the lip, as malignant tumors (48). The reasons for this are the difficulty of image evaluation and the uncertainty of obtaining biopsy findings due to the small size of the lip lesion. Although the masses we observed in Patients 1–3 were palpable and had no adhesions to surrounding tissues, and since the long-term courses of the lesions ruled out malignancy, it is useful to conduct imaging and histological examinations in order to establish a differential diagnosis, even when the tumor is clinically diagnosed as benign.
In addition, because an incisional biopsy involves incision into the capsule and the possibility of cell seeding, surgical treatment with the assumption of removal is considered useful. Notably, many PA lesions on the lips are small in size, and if the resection area is larger than necessary, it may cause postoperative scar contracture, deformity of the lips due to tissue loss, and functional impairment. Therefore, if there are no adhesions or infiltrations with surrounding tissues, total removal as the biopsy procedure is better than over-excision including surrounding healthy tissues.
A malignant transformation of a PA may occur when the surgical resection of a benign PA is incomplete (49,50). In addition, failure to remove the entire capsule increases the possibility of local recurrence and requires long-term follow-up (5,18,50). A review of 31 reports since 2000 (Table 2) revealed that of a total of 35 resected lip PAs, a histopathologic examination enabled the diagnosis of carcinoma ex pleomorphic adenoma (CXPA) in five patients (14%) (18,23-25,40). In one of these patients, additional resection was performed (18), and the remaining four patients were followed up cautiously, considering that they were completely resected (23-25,40). In all 35 patients, there were no recurrences during the follow-up period (including those not described) (Table 2).
In two of our three present patients, we performed only removal with complete inclusion of the capsule, but in the other patient, the capsule was incompletely resected. However, fortunately, to date there has been no recurrence, no deformity of the lips, and no functional impairment in any of the patients. A few reports mentioned a postoperative recurrence of PA, but not much has been published regarding a specific postoperative policy. The recurrence rate of PA is 2–8% (51); some rare recurrences have been identified within a few years after surgery, and some clinicians believe that 5–10 years of observation is not sufficient. For example, in a study by Valstar et al. the 20-year overall recurrence rate of PA was 6.7%, with a median time to first recurrence of 7 years (52).
Few previous PA reports have included a rigorous, long-term follow-up protocol with periodic imaging. PA recurrences rarely cause clinical symptoms such as pain or neurological symptoms, and without periodic imaging during follow-up, the recurrences may remain undetected for a long period of time. Schapher et al. showed that US, a routine examination, allowed the early detection of recurrence even before clinical symptoms appeared (53). Although there is no set view on follow-up intervals, we will continue the long-term follow-up of our PA patients while taking into consideration the characteristics of PAs, which may reoccur years after surgery.
Conclusions
PA is one of the most frequently occurring salivary gland tumors, but it rarely occurs in minor salivary glands and is especially rare in the lips. PAs show a wide variety of pathologies and histologies, and it may not be possible to make a diagnosis of benign or malignant status based on imaging findings or preoperative biopsy materials alone; in addition, for lip PAs, imaging studies and a biopsy may be difficult to perform. Clinicians should consider the possibility of malignancy and postoperative recurrence and perform a resection that includes the capsule and surrounding tissue. It is also necessary to continue a careful follow-up for signs of recurrence.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-308/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-308/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-308/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kessler AT, Bhatt AA. Review of the Major and Minor Salivary Glands, Part 2: Neoplasms and Tumor-like Lesions. J Clin Imaging Sci 2018;8:48. [Crossref] [PubMed]
- Iwanaga J, Nakamura K, Alonso F, et al. Anatomical study of the so-called "retromolar gland": Distinguishing normal anatomy from oral cavity pathology. Clin Anat 2018;31:462-5. [Crossref] [PubMed]
- Shen D, Ono K, Do Q, et al. Clinical anatomy of the inferior labial gland: a narrative review. Gland Surg 2021;10:2284-92. [Crossref] [PubMed]
- Forty MJ, Wake MJ. Pleomorphic salivary adenoma in an adolescent. Br Dent J 2000;188:545-6. [Crossref] [PubMed]
- Neville B. Oral and maxillofacial pathology. 3rd edition. Saunders, 2003:477-80,93-95.
- Rauchi S. Tumoren der kleinen und aberrierenden Speicheldruesen. Arch Geschwulst 1959;14:243-56.
- Chaudhry AP, Vickers RA, Gorlin RJ. Intraoral minor salivary gland tumors. An analysis of 1,414 cases. Oral Surg Oral Med Oral Pathol 1961;14:1194-226. [Crossref] [PubMed]
- Bernier JL. Mixed tumors of the lip. Ann Dent 1945;4:125-30. [PubMed]
- Krolls SO, Hicks JL. Mixed tumors of the lower lip. Oral Surg Oral Med Oral Pathol 1973;35:212-7. [Crossref] [PubMed]
- Lotufo MA, Júnior CA, Mattos JP, et al. Pleomorphic adenoma of the upper lip in a child. J Oral Sci 2008;50:225-8. [Crossref] [PubMed]
- Kataria SP, Tanwar P, Sethi D, et al. Pleomorphic adenoma of the upper lip. J Cutan Aesthet Surg 2011;4:217-9. [Crossref] [PubMed]
- Buchner A, Merrell PW, Carpenter WM. Relative frequency of intra-oral minor salivary gland tumors: a study of 380 cases from northern California and comparison to reports from other parts of the world. J Oral Pathol Med 2007;36:207-14. [Crossref] [PubMed]
- Bhatia JSS. Pleomorphic Adenoma of Upper Lip: A Rare Case Presentation. Indian J Otolaryngol Head Neck Surg 2019;71:755-8. [Crossref] [PubMed]
- To EW, Tsang WM, Tse GM. Pleomorphic adenoma of the lower lip: report of a case. J Oral Maxillofac Surg 2002;60:684-6. [Crossref] [PubMed]
- Jorge J, Pires FR, Alves FA, et al. Juvenile intraoral pleomorphic adenoma: report of five cases and review of the literature. Int J Oral Maxillofac Surg 2002;31:273-5. [Crossref] [PubMed]
- Moritani N, Yamada T, Mishima K, et al. Two cases of pleomorphic adenoma of the lip. Japanese Journal of Oral & Maxillofacial Surgery 2008;54:688-92. [Crossref]
- Asuquo ME, Otei OO, Ekpo R, et al. Salivary gland tumour of the lip: report of two cases and literature review. Cent Afr J Med 2009;55:43-6. [PubMed]
- McNamara ZJ, Batstone M, Farah CS. Carcinoma ex pleomorphic adenoma in a minor salivary gland of the upper lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e51-3. [Crossref] [PubMed]
- Debnath SC, Adhyapok AK. Pleomorphic adenoma (benign mixed tumour) of the minor salivary glands of the upper lip. J Maxillofac Oral Surg 2010;9:205-8. [Crossref] [PubMed]
- Shrestha A, Reddy NS, Ganguly SN. Pleomorphic adenoma of the upper lip: A case report. Journal of College of Medical Sciences-Nepal 2010;6:51-3. [Crossref]
- Ali I, Gupta AK, Singh S. Pleomorphic adenoma of the upper lip. Natl J Maxillofac Surg 2011;2:219-21. [Crossref] [PubMed]
- Sengul I, Sengul D, Aribas D. Pleomorphic adenoma of the lower lip: A rare site of location. N Am J Med Sci 2011;3:299-301. [Crossref] [PubMed]
- Dyalram D, Huebner T, Papadimitriou JC, et al. Carcinoma ex pleomorphic adenoma of the upper lip. Int J Oral Maxillofac Surg 2012;41:364-7. [Crossref] [PubMed]
- Mitate E, Kawano S, Kiyoshima T, et al. Carcinoma ex pleomorphic adenoma of the upper lip: a case of an unusual malignant component of squamous cell carcinoma. World J Surg Oncol 2013;11:234. [Crossref] [PubMed]
- Mariano FV, Rincon D, Gondak RO, et al. Carcinoma ex-pleomorphic adenoma of upper lip showing copy number loss of tumor suppressor genes. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:69-74. [Crossref] [PubMed]
- Tzermpos F, Chatzichalepli C, Cocos A, et al. Atypical Presentation of an Upper Lip Pleomorphic Adenoma: Case Report. Acta Stomatol Croat 2014;48:48-53. [Crossref] [PubMed]
- Sood A, Chung S, Datiashvili RO. An incidental finding of pleomorphic adenoma of the minor salivary glands in the skin area of the lower lip. Eplasty 2014;14:e39. [PubMed]
- Fomete B, Adeosun OO, Awelimobor DI, et al. Recurrent pleomorphic adenoma of the upper lip: case report and review of the literature. Niger J Med 2015;24:277-80. [PubMed]
- Singh AK, Kumar N, Sharma P, et al. Pleomorphic adenoma involving minor salivary glands of upper lip: A rare phenomenon. J Cancer Res Ther 2015;11:1025. [Crossref] [PubMed]
- Khan MN, Raza SS, Hussain Zaidi SA, et al. Pleomorphic Adenoma Of Minor Salivary Glands. J Ayub Med Coll Abbottabad 2016;28:620-2. [PubMed]
- Taniguchi K, Naito H, Mori K, et al. A case of pleomorphic adenoma of the lower lip. Japanese Journal of Oral Diagnosis / Oral Medicine 2016;29:86-91.
- Metgud R, Neelesh BT, Goel S, et al. Pleomorphic adenoma of the upper lip: a case report and review of literature. International Journal of Dental Research 2016;4:8. [Crossref]
- Fatahzadeh M. Pleomorphic Adenoma of the Upper Lip: A Rare Case. N Y State Dent J 2017;83:47-51. [PubMed]
- Ahmedi JR, Ahmedi E, Perjuci F, et al. Pleomorphic Adenoma of Minor Salivary Glands in Child. Med Arch 2017;71:360-3. [Crossref] [PubMed]
- Alves VLA, Pérez-de-Oliveira ME, de Castro JFL, et al. Intraoral Pleomorphic Adenoma in Young Patients. J Craniofac Surg 2018;29:e209-11. [Crossref] [PubMed]
- Taiwo AO, Akinshipo A, Braimah RO, et al. Pleomorphic Adenoma of the Upper Lip: A Case Report. Saudi J Med Med Sci 2018;6:32-5. [Crossref] [PubMed]
- Yoshimura S, Nishimura G, Shiozawa K, et al. A Case of Pleomorphic Adenoma of the Lip. Japanese Journal of Plastic Surgery 2018;61.
- Nourwali I, Dar-Odeh N. Pleomorphic Adenoma in the Lower Lip: A Case Report and a Review. Eur J Dent 2019;13:649-53. [Crossref] [PubMed]
- Kazikdas KC, Yalcinozan ET, Dirik MA. Pleomorphic adenoma of the upper lip. Natl J Maxillofac Surg 2020;11:110-2. [Crossref] [PubMed]
- Shome S, Shah N, Mahmud SKA, et al. A miscellany of cribriform pattern, squamous metaplasia and clear cells in pleomorphic adenoma of upper lip: A diagnostic paradox. J Oral Maxillofac Pathol 2020;24:S46-50. [Crossref] [PubMed]
- Adiyodi NV, Sequeira J, Mehra A. Twinning of Pleomorphic Adenoma: A Case Report. Cureus 2020;12:e6608. [Crossref] [PubMed]
- Kamijo Y. Oral Anatomy. Tokyo: Anatom Publisher, 1969.
- Thackray A, Lucas, RB. Adenolymphoma. In: Tumors of the major salivary glands. Washington, DC, USA: Armed Forces Institute of Pathology, 1974:40-55.
- Okui T, Ibaragi S, Fujita M, et al. A Case of Pleomorphic Adenoma Originating from Accessory Parotid Gland. J Maxillofac Oral Surg 2021;20:573-6. [Crossref] [PubMed]
- Hasegawa K, Sukegawa S, Ono S, et al. Endoscopic-assisted resection of pleomorphic adenoma in the accessory parotid gland. J Med Invest 2021;68:376-80. [Crossref] [PubMed]
- Neville BW, Damm DD, Weir JC, et al. Labial salivary gland tumors. Cancer 1988;61:2113-6. [Crossref] [PubMed]
- Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg 2005;63:805-10. [Crossref] [PubMed]
- Takayama T, Ikai A, Hayashi K, et al. Carcinoma ex pleomorphic adenoma without malignant findings upon clinical in the palate. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology 2018;30:286-9. [Crossref]
- Pons Vicente O, Almendros Marqués N, Berini Aytés L, et al. Minor salivary gland tumors: A clinicopathological study of 18 cases. Med Oral Patol Oral Cir Bucal 2008;13:E582-8. [PubMed]
- Choi SY, Choi J, Hwang I, et al. Comparative Longitudinal Analysis of Malignant Transformation in Pleomorphic Adenoma and Recurrent Pleomorphic Adenoma. J Clin Med 2022;11:1808. [Crossref] [PubMed]
- Batsakis JG, Regezi JA, Bloch D. The pathology of head and neck tumors: salivary glands, part 3. Head Neck Surg 1979;1:260-73. [Crossref] [PubMed]
- Valstar MH, de Ridder M, van den Broek EC, et al. Salivary gland pleomorphic adenoma in the Netherlands: A nationwide observational study of primary tumor incidence, malignant transformation, recurrence, and risk factors for recurrence. Oral Oncol 2017;66:93-9. [Crossref] [PubMed]
- Schapher M, Koch M, Goncalves M, et al. Extracapsular Dissection in Pleomorphic Adenomas of the Parotid Gland: Results After 13 Years of Follow-up. Laryngoscope 2021;131:E445-51. [Crossref] [PubMed]


