
Change of treatment modality and outcomes of adrenocortical carcinoma: a retrospective review of single tertiary center experience over 24 years
Introduction
Adrenal incidentalomas are common, developing in 3–10% of the population, in which the majority of tumors are adenomas (1). Conversely, adrenocortical carcinoma (ACC) is an extremely rare malignancy with an incidence of 1 to 2 cases per million (2-4). Although it has no uniform patterns, ACC may be diagnosed based on the size and imaging characteristics of the lesion (5,6). The incidence rate of ACC is highest in those in their 50s and 60s and is higher in women (7,8). Most patients with ACC present poor outcomes, although the prognosis varies greatly depending on the initial tumor stage (9). When the tumor is confined within the adrenal gland, the 5-year survival rate is 58–66% and decreases to 0–24% with extra-adrenal ACC (10,11). For ACC, complete surgical resection, such as en bloc and R0 resection, is the only curative treatment (CTr) associated with a superior prognosis (12-14). However, despite complete resection, the recurrence rate may increase to 75–85% (11,15,16). For this reason, in many studies, adjuvant therapy, including chemotherapy and/or mitotane therapy, is recommended with surgery (11,17-20). Mitotane has been used as drug therapy for unresectable or metastatic ACC since 1959 (21-23). Moreover, it is the only approved agent for treating patients with ACC (20,24). In the early days of the development of mitotane, mitotane monotherapy was the main treatment method for ACC that was difficult to resect completely or had metastasized. According to the results of several studies at that time, the tumor size was reduced in 34–61% of patients with ACC treated with mitotane (23,25,26). However, recent studies have reported partial response rates of 10–30% of patients, and some studies have even shown lower rates (10,27-29). With this, the effectiveness of mitotane for the treatment of ACC has not been well proven, and several studies have shown contradictory results (15,17,30-34).
This study aimed to review the demographic and clinical features of 94 patients with ACC who visited a single tertiary center over the last two decades and evaluate the trend of treatment modality and prognostic outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-274/rc).
Methods
This retrospective cohort study analyzed 109 patients with ACC who visited Asan Medical Center, Seoul, Korea, between January 1995 and June 2020. The cutoff age of the enrolled patients was >18 years. Among them, 15 patients were excluded due to lack of data, and 94 patients were finally enrolled. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea (No. 2015-0376) and informed consent was waived due to the retrospective nature of the study. Clinical information related to the patient was retrospectively collected by an electronic medical information system. Seventy-one patients were diagnosed of ACC through surgery while 17 patients having multiple metastatic lesions underwent percutaneous adrenal biopsy to diagnose the origin of carcinoma. And, 6 patients were diagnosed through image studies only.
First, we analyzed the age, sex, stage, lesion site, tumor size, hormonal production status, operative status, mitotane use, multidisciplinary treatment method, R0 resection, recurrence rate, and disease-free survival (DFS) of patients with ACC. In this study, R0 resection was defined as complete surgical resection with pathologic negative margin. Of the 71 patients who underwent surgery, open surgery was performed on 62 patients, and minimally invasive surgery including laparoscopic or robotic surgery was performed on 9 patients. The DFS period was from the date of complete surgical resection to the date of the first recurrence. In this study, chemotherapy refers to chemotherapy agents other than mitotane, including cytotoxic agents (etoposide, adriamycin, and cisplatin). The ACC stage was classified according to the classification of American Joint Committee on Cancer (AJCC) 8th edition tumor-node-metastasis (TNM) staging (35). Then, the trend of demographic and clinical features of ACC were analyzed by dividing the study duration into 5-year periods. Follow-up studies included computed tomography, magnetic resonance imaging, and/or positron emission tomography images every 3 to 6 months postoperatively. Recurrence was dictated and evaluated when newly developed lesions were found in consecutive imaging studies after R0 resection. In this study, patients who underwent R0 resection were referred to as having CTr and others were considered to have palliative treatments (PTr) or less.
The primary endpoint of this study was the distribution of ACC according to TNM stages and how R0 resection and multidisciplinary treatment were performed. Then, we evaluated the recurrence pattern according to the definition under CTr and PTr. As a second endpoint, we analyzed the changing demographic and clinical outcome trends annually.
Statistical analysis
We assessed the variables using the Pearson chi-square test for categorical variables and the Student’s independent t-test for continuous variables. Continuous variables are presented as mean ± standard deviations with ranges, and categorical variables as percentages and absolute numbers. Statistical analysis was performed using IBM SPSS version 21.0 for Windows (Chicago, IL, USA), and differences were considered significant when a P value was <0.05.
Results
A total of 94 patients were included in this study. The baseline demographic and clinical features are presented in Table 1. The mean age was 48.94±13.61 years (range, 19–78 years). Most patients were aged between 41 and 50 years (n=27, 28.7%), followed by those between 51 and 60 years (n=23, 24.5%). There were five patients aged >70 years. The number of male and female patients was the same (n=47; 50%).
Table 1
| Variables | Total (n=94) | Stage 1 (n=5) | Stage 2 (n=34) | Stage 3 (n=19) | Stage 4 (n=36) | P value‡ |
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 48.94±13.61 | 50.4±5.4 | 49.0±16.1 | 50.1±13.6 | 48.1±12.2 | 0.952 |
| 19–30, n (%) | 9 (9.6) | 0 | 6 (17.6) | 1 (5.3) | 2 (5.6) | 0.343 |
| 31–40, n (%) | 17 (18.1) | 0 | 5 (14.7) | 3 (15.8) | 9 (25.0) | |
| 41–50, n (%) | 27 (28.7) | 3 (60.0) | 8 (23.5) | 5 (26.3) | 11 (30.6) | |
| 51–60, n (%) | 23 (24.5) | 2 (40.0) | 6 (17.6) | 7 (36.8) | 8 (22.2) | |
| 61–70, n (%) | 13 (13.8) | 0 | 6 (17.6) | 1 (5.3) | 6 (16.7) | |
| 71–78, n (%) | 5 (5.3) | 0 | 3 (8.8) | 2 (10.5) | 0 | |
| Sex, n (%) | 0.761 | |||||
| Male | 47 (50.0) | 3 (60.0) | 15 (44.1) | 9 (47.4) | 20 (55.6) | |
| Female | 47 (50.0) | 2 (40.0) | 19 (55.9) | 10 (52.6) | 16 (44.4) | |
| Lesion site, n (%) | 0.876 | |||||
| Right | 42 (44.7) | 2 (40.0) | 13 (38.2) | 8 (42.1) | 19 (52.8) | |
| Left | 50 (53.2) | 3 (60.0) | 20 (58.8) | 11 (57.9) | 16 (44.4) | |
| Bilateral | 2 (2.1) | 0 | 1 (2.9) | 0 | 1 (2.8) | |
| Tumor size, cm (mean ± SD; range, 2.5–28.0) | 11.7±5.1 | 4.9±2.0 | 12.1±5.0 | 13.1±5.3 | 11.6±4.8 | 0.013 |
| Hormonal production, n (%) | 0.140 | |||||
| None | 29 (30.9) | 3 (60.0) | 10 (29.4) | 8 (42.1) | 8 (22.2) | 0.398§ |
| Catecholamine | 2 (2.1) | 0 | 1 (2.9) | 0 | 1 (2.8) | |
| Aldosterone | 4 (4.3) | 1 (20.0) | 1 (2.9) | 1 (5.3) | 1 (2.8) | |
| Cortisol | 23 (24.5) | 0 | 7 (20.6) | 4 (21.1) | 12 (33.3) | |
| Cortisol + aldosterone | 2 (2.1) | 1 (20.0) | 0 | 0 | 1 (2.8) | |
| Not examined | 34 (36.2) | 0 | 15 (44.1) | 6 (31.6) | 13 (36.1) | |
| Operation, n (%) | <0.001 | |||||
| Yes | 71 (75.5) | 5 (100.0) | 33 (97.1) | 14 (73.7) | 19 (52.8) | |
| No | 23 (24.5) | 0 | 1 (2.9)† | 5 (26.3) | 17 (47.2) | |
| Mitotane, n (%) | 0.917 | |||||
| Yes | 54 (57.4) | 3 (60.0) | 20 (58.8) | 9 (47.4) | 22 (61.1) | |
| No | 40 (42.6) | 2 (40.0) | 14 (41.2) | 10 (52.6) | 14 (38.9) | |
| Treatment, n (%) | <0.001 | |||||
| None | 9 (9.6) | 0 | 1 (2.9) | 4 (21.1) | 4 (11.1) | |
| Surgery only | 15 (16.0) | 2 (40.0) | 5 (14.7) | 5 (26.3) | 3 (8.3) | |
| Surgery and adjuvant chemotherapy with mitotane | 46 (49.0) | 3 (60.0) | 20 (58.8) | 8 (42.1) | 15 (41.7) | |
| Surgery and adjuvant chemotherapy without mitotane | 10 (10.6) | 0 | 8 (23.5) | 1 (5.3) | 1 (2.8) | |
| Chemotherapy with mitotane | 8 (8.5) | 0 | 0 | 0 | 6 (16.7) | |
| Chemotherapy without mitotane | 6 (6.4) | 0 | 0 | 1 (5.3) | 7 (19.4) | |
| R0 resection, n (%) | 61 (64.9) | 5 (100.0) | 33 (97.1) | 12 (63.2) | 11 (30.6) | <0.001 |
| Recurrence, n (%) | 0.152 | |||||
| Yes | 38 (62.3) | 2 (40.0) | 19 (57.6) | 7 (58.3) | 10 (90.9) | |
| No | 23 (37.7) | 3 (60.0) | 14 (42.4) | 5 (41.7) | 1 (9.1) | |
| Disease-free survival, months (mean ± SD; range, 0–280) | 37.5±49.8 | 51.6±27.8 | 49.9±60.2 | 24.6±31.5 | 7.8±7.2 | 0.062 |
†, surgery was not possible due to a medical condition not related to adrenocortical carcinoma; ‡, P value means a statistically significant difference between stages; §, P value excluding patients who were not examined. AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; SD, standard deviation.
The mean tumor size was 11.7±5.1 cm (range, 2.5–28.0 cm). Seventy-one patients (75.5%) underwent surgery, and 54 (57.4%) used mitotane during treatment. Most patients (49.0%) underwent multidisciplinary treatment, such as surgery and adjuvant chemotherapy with mitotane, while 16.0% underwent only surgery. PTr such as chemotherapy with/without mitotane was performed in 14.9% of patients with ACC. Overall, nine patients (9.6%) did not receive any treatments. R0 resection was performed in 61 patients (64.9%), and among them, 38 patients (62.3%) presented recurrence during the follow-up period. The mean DFS among patients who received CTr was 37.5±49.8 months.
Table 1 shows the distribution of patients with ACC according to the AJCC 8th edition TNM staging. Among all patients, 36 (38.3%) had stage 4, while 34 (36.2%) had stage 2 and 19 (20.2%) had stage 3. Stage 1 was the least common, with 5 patients (5.3%). There were no significant differences in mean age, age distribution, sex ratio, and tumor lesion ratio according to stages (P>0.05). The mean tumor size was the largest in patients with stage 3 at 13.1±5.3 cm (P=0.013). Adrenal functional hormone test was performed in 60 patients, and 31 presented hormonal production (P=0.140). The proportion of surgeries performed presented significant differences between the stages (P<0.001). Surgery was performed in 100%, 97.1%, 73.7%, and 52.8% of patients with stages 1, 2, 3, and 4, respectively. The proportion of patients administered mitotane for the ACC treatment did not significantly differ according to each stage (P=0.917). There was a statistically significant difference in the multidisciplinary treatment methods for each stage (P<0.001). Radiation treatment was performed in 18 patients (19.1%). In this study, we did not include this as a part of treatment evaluation since the distribution of radiation were broad with very small number in each treatment group. The higher the stage, the higher the PTr rate in which the surgical approach was not applicable. The R0 resection rates were 100% in stages 1 and 97.1% in stage 2, 63.2% in stage 3, and 30.6% in stage 4 (P<0.001). The DFS was not statistically significant but was decreasing with the increasing stage (P=0.062).
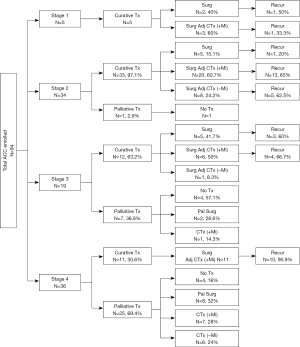
The CTr and PTr rates and recurrences accordingly to each stage are shown in Figure 1. In stage 1, CTr was possible in all five patients. Among them, one of two patients who underwent only surgery and one of three patients who received adjuvant chemotherapy (adj-CTx), including mitotane after surgery, had recurrences. In stage 2, one of 34 patients received PTr, and this patient could not undergo surgery due to severe underlying medical conditions. In case of recurrent ACC in stage 2, 1 of 5 patients (20%) who underwent surgery only, 13 of 20 patients (65%) who underwent adj-CTx with mitotane after surgery, and five of eight patients (62.5%) who underwent adj-CTx without mitotane after surgery showed ACC recurrence. In stage 3, 36.8% of patients underwent PTr, and 41.7% of patients who received CTr underwent surgery only. Moreover, 60% of patients who underwent surgery only and 66.7% of patients who underwent adj-CTx with mitotane after surgery showed recurrence. One patient with stage 3 underwent adj-CTx without mitotane and did not show any recurrence. In stage 4, the proportion of patients who underwent PTr was 69.4%, and adj-CTx, including mitotane, was performed after surgery in all 11 patients who underwent CTr. Of these, ten patients (90.9%) showed recurrence.
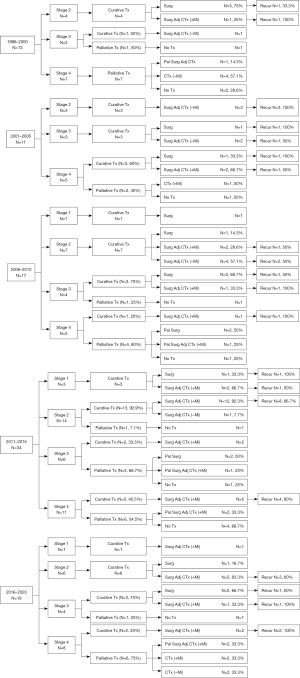
We analyzed the trend of demographic and clinical features of ACC into 5-year units according to the year of diagnosis (Table 2). There was no statistically significant change in the mean age or distribution of age groups (P>0.05). Since 2011, five patients aged >70 years have had ACC. The male-female ratio was maintained at a similar rate with no statistically significant difference (P=0.517). The distribution of ACC stages presented no statistical differences; however, the proportion of patients with stages 3 and 4 was higher than those with stages 1 and 2 in the overall population and the 5-year periods (P=0.950). The mean tumor size did not show a statistically significant difference according to the annual trend (P=0.077). However, the mean tumor size was larger in the 2001–2005 unit and then decreased slightly in every subsequent 5-year unit. Hormone test was actively performed since late 2010. Adrenal functional hormone test was gradually performed since then, and the proportion of hormonal production presented uniform rate (P=0.976). The percentage of patients with ACC who underwent surgery was >70% except for the first 5 years. Mitotane use showed a significant difference according to the 5-year unit (P<0.001). Between 1996 and 2000, only 7.7% underwent mitotane therapy, but it gradually increased thereafter, and during the last 5 years, 68.4% of patients underwent mitotane therapy. The multidisciplinary treatment method showed a significant difference in the periods (P<0.001). In the early periods, mitotane was not used well in addition to adjuvant chemotherapy after surgery or PTr, as mitotane use was observed in only 7.7%. However, the rate of mitotane use increased in the later periods, in which the rates of surgery and adjuvant chemotherapy with mitotane and chemotherapy with mitotane increased up to 57.9% and 10.5%, respectively. The R0 resection and recurrence rates showed no differences throughout the study period (P>0.05). Interestingly, the DFS interval shortened over time (P=0.041).
Table 2
| Variables | 1996–2000 (n=13) | 2001–2005 (n=11) | 2006–2010 (n=17) | 2011–2015 (n=34) | 2016–2020 (n=19) | P value† |
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 46.4±15.4 | 48.5±11.8 | 44.0±10.4 | 53.7±12.5 | 46.8±16.3 | 0.114 |
| 19–30, n (%) | 2 (15.4) | 0 | 2 (11.8) | 2 (5.9) | 3 (15.8) | 0.455 |
| 31–40, n (%) | 3 (23.1) | 3 (27.3) | 4 (23.5) | 3 (8.8) | 4 (21.1) | |
| 41–50, n (%) | 3 (23.1) | 4 (36.4) | 7 (41.2) | 10 (29.4) | 3 (15.8 | |
| 51–60, n (%) | 2 (15.4) | 1 (9.1) | 4 (23.55) | 10 (29.4) | 6 (31.6) | |
| 61–70, n (%) | 3 (23.1) | 3 (27.3) | 0 | 6 (17.6) | 1 (5.3) | |
| 71–78, n (%) | 0 | 0 | 0 | 3 (8.8) | 2 (10.5) | |
| Sex, n (%) | 0.517 | |||||
| Male | 5 (38.5) | 5 (45.5) | 7 (41.2) | 21 (61.8) | 9 (47.4) | |
| Female | 8 (61.5) | 6 (54.5) | 10 (58.8) | 13 (38.2) | 10 (52.6) | |
| Stage, n (%) | 0.950 | |||||
| 1 | 0 | 0 | 1 (5.9) | 3 (8.8) | 1 (5.3) | |
| 2 | 4 (30.8) | 3 (27.3) | 7 (41.2) | 14 (41.2) | 6 (31.6) | |
| 3 | 2 (15.4) | 3 (27.3) | 4 (23.5) | 6 (17.6) | 4 (21.1) | |
| 4 | 7 (53.8) | 5 (45.5) | 5 (29.4) | 11 (32.4) | 8 (42.1) | |
| Lesion site, n (%) | 0.057 | |||||
| Right | 9 (69.2) | 5 (45.5) | 7 (41.2) | 11 (32.4) | 10 (52.6) | |
| Left | 4 (30.8) | 6 (54.5) | 8 (47.1) | 23 (67.6) | 9 (47.4) | |
| Bilateral | 0 | 0 | 2 (11.8) | 0 | 0 | |
| Tumor size, cm (mean ± SD; range, 2.5–28.0) | 11.9±6.7 | 15.0±4.0 | 13.0±4.1 | 10.8±5.4 | 10.2±4.0 | 0.077 |
| Hormonal production, n (%) | 0.002 | |||||
| None | 0 | 0 | 7 (41.2) | 14 (41.2) | 8 (42.1) | 0.976‡ |
| Catecholamine | 1 (7.7) | 0 | 1 (5.9) | 0 | 0 | |
| Alodosterone | 0 | 0 | 0 | 2 (5.9) | 2 (10.5) | |
| Cortisol | 1 (7.7) | 2 (18.2) | 4 (23.5) | 10 (29.4) | 6 31.6) | |
| Cortisol + aldosterone | 0 | 0 | 1 (5.9) | 1 (2.9) | 0 | |
| No examed | 11 (84.6) | 9 (81.8) | 4 (23.5) | 7 (20.6) | 3 (15.8) | |
| Operation, n (%) | 0.088 | |||||
| Yes | 6 (46.2) | 9 (81.8) | 15 (88.2) | 27 (79.4) | 14 (73.7) | |
| No | 7 (53.8) | 2 (18.2) | 2 (11.8) | 7 (20.6) | 5 (26.3) | |
| Mitotane, n (%) | <0.001 | |||||
| Yes | 1 (7.7) | 5 (45.5) | 5 (29.4) | 30 (88.2) | 13 (68.4) | |
| No | 12 (92.3) | 6 (54.5) | 12 (70.6) | 4 (11.8) | 6 (31.6) | |
| Treatment, n (%) | <0.001 | |||||
| None | 3 (23.1) | 1 (9.1) | 2 (11.8) | 2 (5.9) | 1 (5.3) | |
| Surgery only | 3 (23.1) | 2 (18.2) | 6 (35.3) | 1 (2.9) | 3 (15.8) | |
| Surgery and adjuvant chemotherapy with mitotane | 1 (7.7) | 4 (36.4) | 5 (29.4) | 25 (73.5) | 11 (57.9) | |
| Surgery and adjuvant chemotherapy without mitotane | 2 (15.4) | 3 (27.3) | 4 (23.5) | 1 (2.9) | 0 | |
| Chemotherapy with mitotane | 0 | 1 (9.1) | 0 | 5 (14.7) | 2 (10.5) | |
| Chemotherapy without mitotane | 4 (30.8) | 0 | 0 | 0 | 2 (10.5) | |
| R0 resection, n (%) | 5 (38.5) | 9 (81.8) | 12 (70.6) | 23 (67.6) | 12 (63.2) | 0.219 |
| Recurrence, n (%) | 0.117 | |||||
| Yes | 2 (40.0) | 9 (100.0) | 6 (50.0) | 14 (60.9) | 7 (58.3) | |
| No | 3 (60.0) | 0 | 6 (50.0) | 9 (39.1) | 5 (41.7) | |
| Disease-free survival, months (mean ± SD; range, 0–280) |
71.6±118.0 | 8.1±6.5 | 62.8±60.5 | 35.8±32.1 | 23.2±22.7 | 0.041 |
| Follow-up duration, months (mean ± SD; range, 0–280) |
43.6±95.4 | 25.7±29.8 | 54.2±59.5 | 41.3±39.4 | 24.8±23.2 | 0.435 |
†, P value means a statistically significant difference between trends over time; ‡, P value excluding patients who were not examined. SD, standard deviation.
Figure 2 shows the change over time by dividing the data of Figure 1 into 5-year units. In stages 1 and 2, CTr was performed in all except one case where surgery was not performed due to a poor medical condition in the entire period. From 1996 to 2000, CTr was possible in 5 of 13 patients. One of two patients with stage 3 underwent CTr, but there was no recurrence. Between 2001 and 2005, 9 of 11 patients underwent CTr. Of these, three patients in stage 2 received adj-CTx without mitotane after surgery, but all relapsed. In stages 3 and 4, recurrence occurred in two patients who underwent surgery only, and 50% of patients who underwent adj-CTx with or without mitotane after surgery had recurrences. From 2006 to 2010, among patients with stages 1 and 2, two who underwent surgery only did not show recurrence, and 50% of patients who underwent adj-CTx with or without mitotane therapy after surgery had recurrences. Between 2011 and 2015, among 23 patients who underwent CTr, only one underwent surgery only, and the remaining 22 patients underwent adj-CTx with or without mitotane after surgery. From 2016 to 2020, two of three patients who underwent CTr in stage 3 had recurrences, and two patients who underwent CTr in stage 4 had recurrences.
Discussion
We have evaluated the prognostic outcomes and treatment modality trend changes of ACC patients according to the stages and periods. In this study, the mean age at diagnosis of ACC was 48.9 years, and there was no significant change in the mean age throughout the period (P=0.952). These results are similar to those of other previous studies (10,36,37). The sex ratio was equal in this study compared to other previous studies that showed a larger proportion of women (9,10,38,39). Tumor size was not statistically different, although it decreased over time (P=0.077). It may be due to advances in diagnostic technology and early diagnosis. However, as shown in Table 2, there was inconsistency in our study that stage 4 patients were the most in recent years.
As a result of analyzing the trend of ACC over the past 20 years in this study, the largest change was mitotane use. The rate of mitotane use, which was only 7.7% initially, has increased to 68.4% recently (P<0.001). There was a significant increase in the number of cases where mitotane was used as an additional therapy to adjuvant chemotherapy after surgery, from 7.7% to 57.9%. Mitotane is currently the only drug approved for ACC treatment (17,19,20,24,34,40). However, to date, the therapeutic effect of mitotane on ACC has been controversial (31-34). In a retrospective cohort study of 177 patients diagnosed with ACC who underwent radical surgery, the group that used mitotane as an adjuvant treatment after surgery significantly increased recurrence-free survival (RFS) compared to the group that did not use mitotane (17). Another retrospective study of 152 patients with non-metastatic ACC also reported a significant increase in RFS in the group using mitotane after surgery and a low risk of recurrence (41). However, another study that analyzed 105 patients with ACC reported little effect of mitotane in ACC treatment (42).
In this study, the rate of mitotane use significantly increased over time, but the DFS period was rather decreased despite no change in stage distribution, tumor size, and R0 resection rate. Although these results may be due to early detection made possible due to high-quality image studies, they show that the therapeutic effect on ACC has not been proven despite the increased use of mitotane. A study analyzing 602 patients with ACC also reported no change in 5-year survival rates for patients with ACC from 1988 to 2002 (37). Even after mitotane has been widely used clinically in patients with ACC, it does not appear to affect overall survival in patients with ACC (17,33,34).
Therefore, cytotoxic agents, such as etoposide, cisplatin, and streptozocin, have been used together with mitotane for a long time as adj-CTx after surgery, and some studies have reported that this treatment is effective and prolongs progression-free survival (21,43-45). Among them, the most preferred method is the combination of low-dose mitotane with etoposide, doxorubicin, and cisplatin (18,43,46-48). However, studies have also reported that these chemotherapeutic agents do not prolong the overall survival of patients with ACC (49,50). Recently, multiple trials are ongoing to evaluate the role of immune checkpoint inhibitors, such as anti-cytotoxic-T-lymphocyte-associated-antigen 4 (anti-CTLA-4), anti-programmed death-1 (anti-PD-1), and anti-PD-ligand-1 (PD-L1) antibodies (48,51-54). In relation to recurrences, surgical approach such as open abdominal surgery and minimally invasive surgery including laparoscopic and robotic surgery may have impact on recurrences (55). However, in this study, minimally invasive surgery was performed only in stages 1 and 2, and the number of cases was too small to compare the prognosis according to the surgical method.
A limitation of this study is that it has the possibility of selection bias as it is a retrospective study. Additionally, this study was conducted in a single institution. For prognosis, extra-adrenal metastatic lesions were not individually considered related to recurrences and multidisciplinary treatment since it was a retrospective study. Lastly, selection bias may have been included in the patients who received non-surgical treatment such as chemotherapy with mitotane who had no pathologic confirmation. However, we believe that this study is valuable because it provides trend changes in multidisciplinary treatment methods of ACC and those related to prognostic outcomes.
Conclusions
The proportion of patients with ACC and stage distribution did not differ over 24 years in our study. Mitotane use has increased recently; however, the prognostic outcomes were not significantly improved even with the use of mitotane and adjuvant chemotherapy. The recurrence rate was presented in a uniform pattern even after CTr during the study period. ACC still presents an extremely poor prognosis, and further prospective studies are needed for these patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-274/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-274/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-274/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-274/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea (No. 2015-0376) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev 2004;25:309-40. [Crossref] [PubMed]
- Baudin EEndocrine Tumor Board of Gustave Roussy. Adrenocortical carcinoma. Endocrinol Metab Clin North Am 2015;44:411-34. [Crossref] [PubMed]
- Gurzu S, Jung I. Adrenocortical carcinoma: update of clinical features and diagnosis. Global Journal of Oncologists 2013;1:42-9. [Crossref]
- Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 2014;35:282-326. [Crossref] [PubMed]
- Barzon L, Scaroni C, Sonino N, et al. Risk factors and long-term follow-up of adrenal incidentalomas. J Clin Endocrinol Metab 1999;84:520-6. [Crossref] [PubMed]
- Ahmed AA, Thomas AJ, Ganeshan DM, et al. Adrenal cortical carcinoma: pathology, genomics, prognosis, imaging features, and mimics with impact on management. Abdom Radiol (NY) 2020;45:945-63. [Crossref] [PubMed]
- Kebebew E, Reiff E, Duh QY, et al. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg 2006;30:872-8. [Crossref] [PubMed]
- Sharma E, Dahal S, Sharma P, et al. The Characteristics and Trends in Adrenocortical Carcinoma: A United States Population Based Study. J Clin Med Res 2018;10:636-40. [Crossref] [PubMed]
- Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer 2008;113:3130-6. [Crossref] [PubMed]
- Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg 2001;25:891-7. [Crossref] [PubMed]
- Libé R. Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Front Cell Dev Biol 2015;3:45. [Crossref] [PubMed]
- Dackiw AP, Lee JE, Gagel RF, et al. Adrenal cortical carcinoma. World J Surg 2001;25:914-26. [Crossref] [PubMed]
- Lee JE, Berger DH, el-Naggar AK, et al. Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery 1995;118:1090-8. [Crossref] [PubMed]
- Kendrick ML, Lloyd R, Erickson L, et al. Adrenocortical carcinoma: surgical progress or status quo? Arch Surg 2001;136:543-9. [Crossref] [PubMed]
- Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery 1992;112:963-70; discussion 970-1. [PubMed]
- Stojadinovic A, Ghossein RA, Hoos A, et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J Clin Oncol 2002;20:941-50. [Crossref] [PubMed]
- Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 2007;356:2372-80. [Crossref] [PubMed]
- Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer 2005;12:657-66. [Crossref] [PubMed]
- Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer 2005;12:667-80. [Crossref] [PubMed]
- Veytsman I, Nieman L, Fojo T. Management of endocrine manifestations and the use of mitotane as a chemotherapeutic agent for adrenocortical carcinoma. J Clin Oncol 2009;27:4619-29. [Crossref] [PubMed]
- Baudin E, Leboulleux S, Al Ghuzlan A, et al. Therapeutic management of advanced adrenocortical carcinoma: what do we know in 2011? Horm Cancer 2011;2:363-71. [Crossref] [PubMed]
- Fassnacht M, Libé R, Kroiss M, et al. Adrenocortical carcinoma: a clinician's update. Nat Rev Endocrinol 2011;7:323-35. [Crossref] [PubMed]
- Bergenstal DM, Hertz R. Chemotherapy of adrenocortical cancer with o, p′ DDD. Ann Intern Med 1960;53:672-82. [Crossref]
- Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2018;179:G1-46. [Crossref] [PubMed]
- Hutter AM Jr, Kayhoe DE. Adrenal cortical carcinoma. Results of treatment with o,p'DDD in 138 patients. Am J Med 1966;41:581-92. [Crossref] [PubMed]
- Lubitz JA, Freeman L, Okun R. Mitotane use in inoperable adrenal cortical carcinoma. JAMA 1973;223:1109-12. [Crossref] [PubMed]
- Baudin E, Pellegriti G, Bonnay M, et al. Impact of monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p'DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer 2001;92:1385-92. [Crossref] [PubMed]
- Haak HR, Hermans J, van de Velde CJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer 1994;69:947-51. [Crossref] [PubMed]
- Barzon L, Fallo F, Sonino N, et al. Adrenocortical carcinoma: experience in 45 patients. Oncology 1997;54:490-6. [Crossref] [PubMed]
- Venkatesh S, Hickey RC, Sellin RV, et al. Adrenal cortical carcinoma. Cancer 1989;64:765-9. [Crossref] [PubMed]
- Puglisi S, Calabrese A, Basile V, et al. New perspectives for mitotane treatment of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab 2020;34:101415. [Crossref] [PubMed]
- Assié G, Antoni G, Tissier F, et al. Prognostic parameters of metastatic adrenocortical carcinoma. J Clin Endocrinol Metab 2007;92:148-54. [Crossref] [PubMed]
- Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol 2010;17:263-70. [Crossref] [PubMed]
- Corso CR, Acco A, Bach C, et al. Pharmacological profile and effects of mitotane in adrenocortical carcinoma. Br J Clin Pharmacol 2021;87:2698-710. [Crossref] [PubMed]
- Amin M, Greene F, Edge S, et al. DP: AJCC Cancer Staging Manual. New York: Springer; 2017.
- Nader S, Hickey RC, Sellin RV, et al. Adrenal cortical carcinoma. A study of 77 cases. Cancer 1983;52:707-11. [Crossref] [PubMed]
- Paton BL, Novitsky YW, Zerey M, et al. Outcomes of adrenal cortical carcinoma in the United States. Surgery 2006;140:914-20; discussion 919-20. [Crossref] [PubMed]
- Kasperlik-Załuska AA, Migdalska BM, Zgliczyński S, et al. Adrenocortical carcinoma. A clinical study and treatment results of 52 patients. Cancer 1995;75:2587-91. [Crossref] [PubMed]
- Henley DJ, van Heerden JA, Grant CS, et al. Adrenal cortical carcinoma--a continuing challenge. Surgery 1983;94:926-31. [PubMed]
- Waszut U, Szyszka P, Dworakowska D. Understanding mitotane mode of action. J Physiol Pharmacol 2017;68:13-26. [PubMed]
- Calabrese A, Basile V, Puglisi S, et al. Adjuvant mitotane therapy is beneficial in non-metastatic adrenocortical carcinoma at high risk of recurrence. Eur J Endocrinol 2019;180:387-96. [Crossref] [PubMed]
- Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med 1990;322:1195-201. [Crossref] [PubMed]
- Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 2012;366:2189-97. [Crossref] [PubMed]
- Gagliano T, Gentilin E, Benfini K, et al. Mitotane enhances doxorubicin cytotoxic activity by inhibiting P-gp in human adrenocortical carcinoma cells. Endocrine 2014;47:943-51. [Crossref] [PubMed]
- Kimpel O, Bedrose S, Megerle F, et al. Adjuvant platinum-based chemotherapy in radically resected adrenocortical carcinoma: a cohort study. Br J Cancer 2021;125:1233-8. [Crossref] [PubMed]
- Berruti A, Terzolo M, Pia A, et al. Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. Cancer 1998;83:2194-200. [Crossref] [PubMed]
- Tacon LJ, Prichard RS, Soon PS, et al. Current and emerging therapies for advanced adrenocortical carcinoma. Oncologist 2011;16:36-48. [Crossref] [PubMed]
- De Filpo G, Mannelli M, Canu L. Adrenocortical carcinoma: current treatment options. Curr Opin Oncol 2021;33:16-22. [Crossref] [PubMed]
- Al Asadi A, Hubbs DM, Sweigert PJ, et al. Analysis of adjuvant chemotherapy in patients undergoing curative-intent resection of localized adrenocortical carcinoma. Am J Surg 2021;222:119-25. [Crossref] [PubMed]
- Kerkhofs TM, Verhoeven RH, Van der Zwan JM, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer 2013;49:2579-86. [Crossref] [PubMed]
- Varghese J, Habra MA. Update on adrenocortical carcinoma management and future directions. Curr Opin Endocrinol Diabetes Obes 2017;24:208-14. [Crossref] [PubMed]
- Bedrose S, Daher M, Altameemi L, et al. Adjuvant Therapy in Adrenocortical Carcinoma: Reflections and Future Directions. Cancers (Basel) 2020;12:508. [Crossref] [PubMed]
- Fiorentini C, Grisanti S, Cosentini D, et al. Molecular Drivers of Potential Immunotherapy Failure in Adrenocortical Carcinoma. J Oncol 2019;2019:6072863. [Crossref] [PubMed]
- Karwacka I, Obołończyk Ł, Kaniuka-Jakubowska S, et al. The Role of Immunotherapy in the Treatment of Adrenocortical Carcinoma. Biomedicines 2021;9:98. [Crossref] [PubMed]
- Buller DM, Hennessey AM, Ristau BT. Open versus minimally invasive surgery for suspected adrenocortical carcinoma. Transl Androl Urol 2021;10:2246-63. [Crossref] [PubMed]


