
Predictive value of the systemic immune-inflammation index for the efficacy of neoadjuvant chemotherapy and prognosis in patients with stage III ovarian cancer—a retrospective cohort study
Introduction
Ovarian cancer is a common malignant tumor of the reproductive system in women. It was reported that the incidence rate of ovarian cancer was 3.6/100,000 to 9.7/100,000, and the 1-, 3-, and 5-year survival rates after diagnosis were 79.7%, 69.7%, and 61.4% respectively (1). Because early ovarian cancer cannot be detected by clinical symptoms, some patients have locally advanced disease at the first diagnosis. Stage III ovarian cancer is defined as a disease with abdominal cavity or retroperitoneal lymph nodes metastasis and liver surface metastasis accompanied by a poor prognosis. Hence, reducing the mortality of these patients is key to reducing the overall mortality of ovarian cancer patients. At present, patients with stage III ovarian cancer are often treated with neoadjuvant chemotherapy combined with interval debulking surgery (2-4). Chemotherapy has a significant curative effect on multiple malignant tumors (5,6), and patients who achieve complete response after neoadjuvant chemotherapy have better outcomes. Therefore, it is of great importance to predict the effectiveness of neoadjuvant chemotherapy in patients with ovarian cancer. CA125 is a common biomarker in patients with ovarian cancer, which is of certain significance in diagnosis and prognosis. However, recent research showed that there was no significant correlation between CA125 and the efficacy of neoadjuvant chemotherapy in patients with ovarian cancer (7), necessitating the identification of biomarkers to predict the efficacy of neoadjuvant chemotherapy in ovarian cancer. The systemic immune-inflammation index (SII), reflecting the immune and inflammatory status of patients with malignant tumors, has been used in the diagnosis and treatment of a variety of malignant tumors and has been found to be related to the prognosis of patients (8-10). Studies also confirmed that a high SII was associated with shortened progression-free survival and increased mortality in patients with ovarian cancer (11). The SII is a predictor of the efficacy of neoadjuvant chemotherapy in patients with gastric cancer, cervical cancer, and breast cancer (12-17). However, there are no studies on the correlation between the SII and the efficacy of neoadjuvant chemotherapy in patients with ovarian cancer. The purpose of our study was to explore the predictive value of the SII for the efficacy of neoadjuvant chemotherapy in patients with stage III ovarian cancer. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-459/rc).
Methods
General information
A total of 102 patients with stage III ovarian cancer treated in Tongji Hospital of Tongji University from January 2017 to January 2019 were retrospectively collected (a retrospective cohort study). According to the level of the SII before neoadjuvant chemotherapy, patients were divided into the high SII group and low SII group. The inclusion criteria were as follows: (I) stage III epithelial ovarian cancer; (II) 18 to 75 years old; (III) patients received neoadjuvant chemotherapy with interval debulking surgery; (IV) Karnofsky performance status score ≥70; (V) patients with completed clinical materials; (VI) patients with measurable target lesions which could evaluate the efficacy of neoadjuvant chemotherapy. The exclusion criteria were as follows: (I) combined with other malignant tumors; (II) liver, kidney, heart, brain, lung, or other organ dysfunction; (III) metastatic ovarian cancer or postoperative recurrence of ovarian cancer; (IV) patients who received other special treatment, such as immunotherapy, anti-angiogenesis agents; (V) patients who dropped out during follow-up. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Tongji Hospital of Tongji University (No. 2022-05-04-012). Individual consent for this retrospective analysis was waived.
Study variables
- The Response Evaluation Criteria in Solid Tumors (RECIST 1.1): The efficacy of neoadjuvant chemotherapy was evaluated by RECIST 1.1, and was divided into complete response, partial response, stable disease, and progressive disease. Complete response was defined as the disappearance of all target lesions, and the short diameter of lymph nodes was <10 mm pathologically. Partial response was defined as the total length of all target lesions decreasing by at least 30% compared with the baseline level. Stable disease was defined as the efficacy of neoadjuvant chemotherapy between partial response and progressive disease. Progressive disease was diagnosed when the total length of all target lesions increased by at least 20% compared with the baseline level and the absolute value increased by at least 5 mm, or new lesions appeared.
- Classification of neoadjuvant chemotherapy efficacy. Efficacy included complete response and partial response, while ineffectiveness included stable disease and progressive disease.
- Progression-free survival. After neoadjuvant chemotherapy, interval debulking surgery was performed. The time from the operation to tumor progression, recurrence, metastasis, or death was defined as progression-free survival.
- The rate of 3-year overall survival. All patients were followed up for 3 years after the operation, and the overall survival rate was observed.
- Age, ascites, menopause status, serum CA125, histological grade, and Federation International of Gynecology and Obstetrics (FIGO) stage were also studied.
Therapeutic strategy
All patients received neoadjuvant chemotherapy with platinum and paclitaxel after admission every 21 days. After 6 cycles of treatment, interval debulking surgery was performed, and adjuvant chemotherapy was subsequently administered.
Statistical analysis
All statistical analyses were performed using SPSS 26.0. A two-tailed P<0.05 was considered statistically significant. Measurement data were expressed as mean ± standard deviation. The independent samples t-test was applied for the comparison of measurement data between 2 groups, and the Kruskal-Wallis test was used for multiple group comparisons. Enumeration data expressed as n (%) was analyzed by the Pearson χ2 test in the comparison of measurement data between 2 groups. Kaplan-Meier analysis was performed to evaluate progression-free survival for the 2 groups. A receiver operating characteristic (ROC) curve was used to analyze the predictive value of the SII and CA125 for postoperative death in ovarian cancer patients. We used multivariate regression analysis to explore the risk factors of death after neoadjuvant chemotherapy in patients with ovarian cancer.
Results
Comparison of general information between the low SII group and high SII group
There was no significant difference between the 2 groups in terms of age, menopause status, FIGO stage, histological grade, and ascites (P>0.05). Compared with the low SII group, the serum CA125 level in the high SII group was significantly higher (914.84±391.64 vs. 756.39±396.21 U/mL, P=0.045) and the SII level increased as well when compared with that of the low SII group (872.78±191.55 vs. 457.87±116.66, P<0.001) (Table 1).
Table 1
| Characteristics | High SII group (n=51) | Low SII group (n=51) | t/χ2 | P value |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 56.25±10.93 | 57.25±10.37 | 0.474 | 0.637 |
| Menopause, n (%) | 0.671 | 0.413 | ||
| Yes | 30 (58.82) | 34 (66.67) | ||
| No | 21 (41.18) | 17 (33.33) | ||
| FIGO stage, n (%) | 3.904 | 0.142 | ||
| IIIA | 21 (41.18) | 12 (23.53) | ||
| IIIB | 15 (29.41) | 22 (43.14) | ||
| IIIC | 15 (29.41) | 17 (33.33) | ||
| Histological grade, n (%) | 0.039 | 0.843 | ||
| Moderately and poorly | 26 (50.98) | 27 (52.94) | ||
| Differentiated, n (%) | ||||
| Well-differentiated | 25 (49.02) | 24 (47.06) | ||
| Ascites, n (%) | 1.325 | 0.250 | ||
| Yes | 42 (82.35) | 46 (90.20) | ||
| No | 9 (17.65) | 5 (9.80) | ||
| CA125 (U/mL) (mean ± SD) | 914.84±391.64 | 756.39±396.21 | 2.031 | 0.045 |
| SII level (mean ± SD) | 872.78±191.55 | 457.87±116.66 | 13.212 | <0.001 |
SII, systemic immune-inflammation index; FIGO, Federation International of Gynecology and Obstetrics.
Correlation between the SII and neoadjuvant chemotherapy efficacy
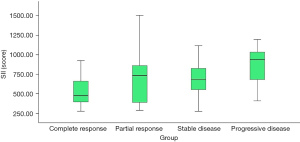
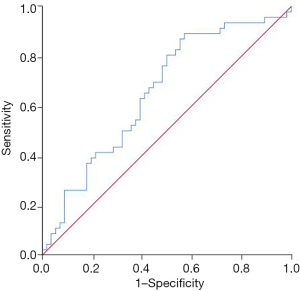
Compared with patients in the low SII group, the complete response rate of patients in the high SII group decreased significantly after neoadjuvant chemotherapy (13.73% vs. 45.10%, P=0.001), and the progressive disease rate increased (19.61% vs. 1.96%, P=0.011) (Table 2). Patients who achieved complete response after neoadjuvant chemotherapy had a lower SII, while patients with progressive disease had a higher SII (P=0.003) (Figure 1). The SII had certain value in predicting the inefficacy of neoadjuvant chemotherapy in patients with stage III ovarian cancer, and the area under the curve was 0.655 (95% CI: 0.548–0.762, P=0.007) (Figure 2).
Table 2
| Neoadjuvant chemotherapy efficacy | High SII group (n=51) | Low SII group (n=51) | χ2 | P value |
|---|---|---|---|---|
| Complete response, n (%) | 7 (13.73) | 23 (45.10) | 16.769 | 0.001 |
| Partial response, n (%) | 15 (29.41) | 11 (21.57) | – | – |
| Stable disease, n (%) | 19 (37.25) | 16 (31.37) | – | – |
| Progressive disease, n (%) | 10 (19.61) | 1 (1.96) | 6.521 | 0.011 |
SII, systemic immune-inflammation index.
Correlation between the SII and progression-free survival in stage III ovarian cancer patients after neoadjuvant chemotherapy
The progression-free survival of patients in the high SII group was shorter than that of patients in the low SII group (P<0.001) (Figure 3).

Correlation between the SII and 3-year overall survival in stage III ovarian cancer patients after neoadjuvant chemotherapy
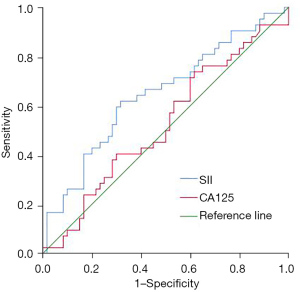
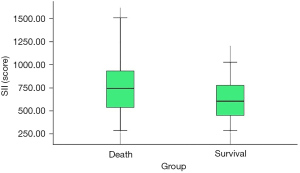
The overall survival rate of patients in the high SII group was lower than that of patients in the low SII group (47.06% vs. 70.59%, P=0.016). The SII had diagnostic value for the postoperative death of ovarian cancer patients after neoadjuvant chemotherapy, and the area under the curve was 0.646 (95% CI: 0.537–0.756, P=0.012). CA125 had no significant predictive value for the postoperative death of ovarian cancer patients after neoadjuvant chemotherapy (P=0.671) (Table 3 and Figure 4). Compared with patients who survived for 3 years after the operation, the SII of patients who died within 3 years after the operation was significantly higher (739.75±269.44 vs. 613.22±244.70, P=0.015) (Figure 5). Multivariate regression analysis showed that higher SII was a risk factor for death in ovarian cancer patients after neoadjuvant chemotherapy (OR: 2.700, P=0.017).
Table 3
| Test result variable(s) | Area | Std. Error | P value | 95% confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| SII | 0.646 | 0.056 | 0.012 | 0.537 | 0.756 |
| CA125 | 0.525 | 0.058 | 0.671 | 0.410 | 0.639 |
SII, systemic immune-inflammation index.
Correlation between neoadjuvant chemotherapy efficacy and progression-free survival
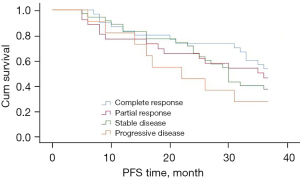
The progression-free survival of patients who achieved complete response after neoadjuvant chemotherapy tended to be longer, but the difference did not reach statistical significance (P=0.302) (Figure 6).
Discussion
There are still few studies exploring the relationship between the SII and the efficacy of neoadjuvant chemotherapy in patients with ovarian cancer. We illustrated the correlation between the SII and the efficacy of neoadjuvant chemotherapy, progression-free survival, and overall survival in patients with ovarian cancer. The results showed that a high SII was associated with inefficacy of neoadjuvant chemotherapy, shortened progression-free survival, and reduced overall survival in patients with ovarian cancer.
Ovarian cancer patients with advanced disease at the first diagnosis often have a poor prognosis. These patients often received neoadjuvant chemotherapy, which can help patients achieve better outcomes. However, some patients have a poor response to neoadjuvant chemotherapy, and even develop progressive disease. These patients then have to undergo intensive treatment. Therefore, it is of great significance to predict the efficacy of neoadjuvant chemotherapy in patients with ovarian cancer before neoadjuvant chemotherapy. Due to the unremarkable value of CA125 in predicting the efficacy of neoadjuvant chemotherapy in patients with ovarian cancer, there are studies on seeking new biomarkers for predicting the efficacy of neoadjuvant chemotherapy in patients with ovarian cancer. Studies showed that the ratio of neutrophils to lymphocytes had a certain value in predicting the efficacy of neoadjuvant chemotherapy in patients with ovarian cancer. Patients with an increased ratio of neutrophils to lymphocytes tended to have a poorer response to neoadjuvant chemotherapy and poor prognosis (18-20). A high ratio of neutrophils to lymphocytes indicates that the level of systemic inflammation is increasing, and the number of lymphocytes killing tumor cells is decreasing. A high level of systemic inflammation can promote the proliferation and metastasis of tumor cells, leading to poor prognosis. Lymphocytes are powerful immune cells that kill tumor cells. A decreased level of lymphocytes indicates that the ability of the body to kill tumor cells is reduced. Recently, the concept of the SII has been proposed, which comprehensively takes the influence of platelets into consideration based on the ratio of neutrophils to lymphocytes. Platelets are small pieces of cytoplasm that detach from the cytoplasm of mature megakaryocytes in bone marrow, and participate in tumor growth, tumor cell extravasation, tumor metastasis, which can inhibit tumor cell apoptosis and maintain the integrity of tumor blood vessels (21-23). Previous studies showed that the progression-free survival and overall survival of ovarian cancer patients with a high SII (>612) were shortened, and a high level of SII was an independent factor resulting in poor prognosis (11). These results support our study. Our results showed that ovarian cancer patients with an increased SII had shorter progression-free survival and a lower 3-year overall survival rate. In addition, the SII level was related to the efficacy of neoadjuvant chemotherapy in patients with stage III ovarian cancer, which was of certain value in predicting the efficacy of neoadjuvant chemotherapy. This suggests that for patients with a high SII, neoadjuvant chemotherapy may need to be further adjusted to improve efficacy and ultimately achieve a better prognosis of ovarian cancer patients.
Limitations
This study was a retrospective clinical study. Only stage III ovarian cancer patients were included, so the sample size was relatively small. This was the deficiency of our study.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-459/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-459/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-459/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Tongji Hospital of Tongji University (No. 2022-05-04-012). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leong E, Ong SK, Jali F, et al. Incidence, Mortality and Survival Analysis of Epithelial Ovarian Cancer in Brunei Darussalam. Asian Pac J Cancer Prev 2022;23:1415-23. [Crossref] [PubMed]
- Mou D, Xie S, Li P. The Evaluation Value of CT in the Efficacy of Neoadjuvant Chemotherapy in Ovarian Cancer Patients. Contrast Media Mol Imaging 2022;2022:7195888. [Crossref] [PubMed]
- Wang H, Fan L, Wu X, et al. Efficacy evaluation of albumin-bound paclitaxel combined with carboplatin as neoadjuvant chemotherapy for primary epithelial ovarian cancer. BMC Womens Health 2022;22:224. [Crossref] [PubMed]
- Hou YM, Xue Y, Yao JM, et al. Relationship Between Neoadjuvant Chemotherapy and Log Odds of Positive Lymph Nodes and Their Prognostic Role in Advanced Ovarian Cancer Patients With Optimal Cytoreductive Surgery. Front Oncol 2022;12:878275. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of anthracycline-based postoperative chemotherapy on blood glucose and lipid profiles in patients with invasive breast cancer. Ann Palliat Med 2021;10:5502-8. [Crossref] [PubMed]
- Alegría-Baños JA, Jiménez-López JC, Vergara-Castañeda A, et al. Kinetics of HE4 and CA125 as prognosis biomarkers during neoadjuvant chemotherapy in advanced epithelial ovarian cancer. J Ovarian Res 2021;14:96. [Crossref] [PubMed]
- Han R, Tian Z, Jiang Y, et al. Prognostic significance of systemic immune-inflammation index and platelet-albumin-bilirubin grade in patients with pancreatic cancer undergoing radical surgery. Gland Surg 2022;11:576-87. [Crossref] [PubMed]
- Xu S, Cao S, Yu Y. High systemic immune-inflammation index is a predictor of poor prognosis in patients with nonsmall cell lung cancer and bone metastasis. J Cancer Res Ther 2021;17:1636-42. [Crossref] [PubMed]
- Zhu M, Chen L, Kong X, et al. The Systemic Immune-Inflammation Index is an Independent Predictor of Survival in Breast Cancer Patients. Cancer Manag Res 2022;14:775-820. [Crossref] [PubMed]
- Nie D, Gong H, Mao X, et al. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: A retrospective study. Gynecol Oncol 2019;152:259-64. [Crossref] [PubMed]
- Liu P, Jiang Y, Zheng X, et al. Pretreatment Systemic Immune-Inflammation Index Can Predict Response to Neoadjuvant Chemotherapy in Cervical Cancer at Stages IB2-IIB. Pathol Oncol Res 2022;28:1610294. [Crossref] [PubMed]
- Demircan NC, Atcı MM, Demir M, et al. Dynamic changes in systemic immune-inflammation index predict pathological tumor response and overall survival in patients with gastric or gastroesophageal junction cancer receiving neoadjuvant chemotherapy. Asia Pac J Clin Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Pang J, Wang S, Liao L, et al. Association between systemic immune-inflammation index and neoadjuvant chemotherapy efficacy as well as prognosis in triple-negative breast cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2021;46:958-65. [PubMed]
- Jiang C, Lu Y, Zhang S, et al. Systemic Immune-Inflammation Index Is Superior to Neutrophil to Lymphocyte Ratio in Prognostic Assessment of Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Biomed Res Int 2020;2020:7961568. [Crossref] [PubMed]
- Chen L, Kong X, Wang Z, et al. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med 2020;24:2993-3021. [Crossref] [PubMed]
- Chen L, Yan Y, Zhu L, et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res 2017;9:849-67. [Crossref] [PubMed]
- Liontos M, Andrikopoulou A, Koutsoukos K, et al. Neutrophil-to-lymphocyte ratio and chemotherapy response score as prognostic markers in ovarian cancer patients treated with neoadjuvant chemotherapy. J Ovarian Res 2021;14:148. [Crossref] [PubMed]
- Sanna E, Tanca L, Cherchi C, et al. Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer. Diagnostics (Basel) 2021;11:1298. [Crossref] [PubMed]
- Kim YJ, Lee I, Chung YS, et al. Pretreatment neutrophil-to-lymphocyte ratio and its dynamic change during neoadjuvant chemotherapy as poor prognostic factors in advanced ovarian cancer. Obstet Gynecol Sci 2018;61:227-34. [Crossref] [PubMed]
- Hu Q, Hada A, Han L. Platelet count as a biomarker for monitoring treatment response and disease recurrence in recurrent epithelial ovarian cancer. J Ovarian Res 2020;13:78. [Crossref] [PubMed]
- Chon S, Lee S, Jeong D, et al. Elevated platelet lymphocyte ratio is a poor prognostic factor in advanced epithelial ovarian cancer. J Gynecol Obstet Hum Reprod 2021;50:101849. [Crossref] [PubMed]
- Qin L, Li JY, Huang WJ, et al. Higher platelet distribution width is associated with unfavorable prognosis in ovarian cancer. Cancer Biomark 2020;28:365-70. [Crossref] [PubMed]


