
Defects of endoscopic biopsy in the diagnosis of periampullary carcinoma and recommendations for diagnosis and treatment: a retrospective study before and after surgery
Introduction
Periampullary carcinoma (PAC), which comprises ampulla of Vater cancer, pancreatic head cancer, distal common bile duct cancer, and duodenal papillary cancer, is a malignant tumor originating from within 2 cm of the ampulla of Vater (1,2) and accounts for 5% of all gastrointestinal tract malignancies (3).
Patients with early-stage PAC are thought to have a better prognosis. The median overall survival in patients with stage I, II, and III PAC has been reported as unreached, 44 months, 15 months, respectively, in a study with a median follow-up of 88 months (4). However, metastasis and advanced stage are considered indicators of poor prognosis in PAC, with a 2-year overall survival rate ranging from 5% to 10% (3,5). Therefore, early diagnosis and treatment are critical. Currently, pancreaticoduodenectomy (PD) is the primary curative treatment for PAC (6), but it is high risk and accompanied by many complications (7). A transatlantic analysis indicated that major complications occurred in 6,188 of 22,983 patients (26.9%) after PD, and 504 (8.1%) patients died in hospital after the development of a major complication, which was defined as failure to rescue. In that study, complications included death, postoperative pancreatic fistula, pneumonia, delayed gastric emptying, organ failure, and reoperation, with an incidence of 2.2%, 12.1%, 5.2%, 15.7%, 6.9%, and 8.2%, respectively (7). The high surgical risk and complication incidence make it difficult to decide whether surgery is necessary, especially for patients with an uncertain preoperative diagnosis. Therefore, accurate preoperative diagnosis and evaluation are essential, as the management of benign lesions can be conservative to avoid the high risks of surgery (8-11).
For the preoperative diagnosis, assessment, and staging of PAC, imaging methods, such as ultrasound (US), computed tomography, magnetic resonance cholangiopancreatography (MRCP), esophagogastroduodenoscopy, endoscopic ultrasonography (EUS), endoscopic retrograde cholangiopancreatography (ERCP), intraductal ultrasound (IDUS), and laboratory investigations, such as tumor markers (CA19-9, CEA, and CA125), are suggested (3,12,13). However, the definitive diagnosis and staging of periampullary tumors still rely on biopsy, and ERCP and EUS as the two common imaging methods used in biopsy (14-16). Despite progress in biopsy, the false-negative rate has remained high for preoperative diagnosis following endoscopic biopsy, which ranges from 11.7% to 60% (15,17-21). The high false-negative rate in patients represents loss of optimal timing of operation and even loss of operative opportunity. Therefore, studies on reducing the false-negative rate and increasing the accuracy of preoperative diagnosis are crucial.
Consequently, we undertook a retrospective single-center study to explore the degree of matching between preoperative and postoperative pathological diagnoses of periampullary tumors, analyze the defects of endoscopic biopsy, and provide recommendations for the diagnosis and treatment of periampullary tumors. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-412/rc).
Methods
This is a retrospective study to explore the diagnostic accuracy of preoperative endoscopic biopsy. From June 2013 to February 2021, 262 patients diagnosed with periampullary tumors (PAC and benign lesions) underwent PD at The Second Affiliated Hospital of Nanchang University. We retrospectively reviewed the data on disease characteristics, pathological diagnosis, and surgical outcomes of each patient, such as sex, age, total bilirubin (TBIL), direct bilirubin (DBIL), tumor markers, imaging features, and preoperative and postoperative pathology. The preoperative pathological diagnosis was based on endoscopic biopsy. The postoperative pathology was based on paraffin sections of the tissues obtained during surgery. PD was the sole surgical option in all patients.
Imaging examination showed that all 262 patients had enlarged papillae and occupying lesions in the papilla of Vater. Following an endoscopic biopsy, occupational lesions were diagnosed as adenocarcinoma, inflammatory non-neoplastic lesions, or dysplasia. We emphasized comparison of the preoperative and final pathology data to determine the difference and degree of matching. After excluding 64 patients who did not undergo preoperative pathological biopsy, 198 were enrolled in the analysis (Figure 1).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of The Second Affiliated Hospital of Nanchang University (No. 2013049). Since this study was retrospective and did not involve personal privacy, informed consent of previous patients could not be obtained objectively.
Statistical analysis
SPSS20.0 software was used. The measurement data with normal distribution are expressed as mean ± standard deviation, and categorical data are expressed as number of cases or percentages.
Results
Preoperative pathology and patients’ characteristics
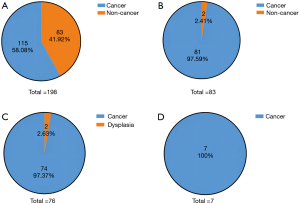
Among the 198 patients in our study, 115 (58.08%) were diagnosed with PAC based on initial preoperative pathology (Figure 2A). The remaining 83 tumors (41.92%) were preoperatively diagnosed as benign and regarded as inflammatory non-neoplastic lesions (n=7) or dysplasia (n=76) (Figure 1, Table S1).
We analyzed the characteristics of the 83 patients diagnosed with benign lesions by preoperative biopsy (Table 1). Their median age was 60 years (range, 35–78 years). The cohort comprised 46 men (55.42%) and 37 women (44.58%). TBIL and DBIL levels were tested in 78 patients: 69.23% (n=54) and 88.46% (n=69) showed increased levels of TBIL and DBIL, respectively. Preoperative and postoperative pathology results were obtained for all 83 patients, with 76 patients who undergoing one preoperative biopsy and 7 patients undergoing two biopsies. The lymph node status was observed in 81 patients.
Table 1
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| Male | 46 | 55.42 |
| Female | 37 | 44.58 |
| Age, years | ||
| Median | 60 | |
| Range | 35–78 | |
| BIL | ||
| TBIL (>23 µmol/L) | 54/78 | 69.23 |
| DBIL (>4 µmol/L) | 69/78 | 88.46 |
| Tumor markers | ||
| CA19-9 (>37 U/mL) | 37/82 | 45.12 |
| CA125 (>35 U/mL) | 4/39 | 10.26 |
| CEA (>5 ng/mL) | 5 | 6.02 |
| Times of preoperative biopsies | ||
| 1 | 76 | 91.57 |
| 2 | 7 | 8.43 |
| Preoperative pathological biopsy | ||
| Dysplasia | 76 | 91.57 |
| Inflammation | 7 | 8.43 |
| Postoperative pathological biopsy | ||
| Cancer | 81 | 97.59 |
| Dysplasia | 2 | 2.41 |
| Lymph node status | ||
| Positive | 16/81 | 19.75 |
| Negative | 65/81 | 80.25 |
| Pathologic stage (AJCC 8) | ||
| I | 38/79 | 48.10 |
| II | 25/79 | 31.65 |
| III | 16/79 | 20.25 |
BIL, bilirubin; TBIL, total bilirubin; DBIL, direct bilirubin; AJCC, American Joint Committee on Cancer.
Postoperative pathology
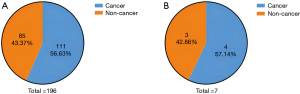
Of the whole cohort (n=198), 115 patients in the PAC subgroup were still diagnosed with PAC based on postoperative pathology results, which aligned with their preoperative biopsy results. However, among the 83 patients diagnosed with benign lesions on preoperative biopsy, 81 (97.59%) had different results for postoperative pathology and were ultimately diagnosed with PAC (Figure 2B). In the other two patients (2.41%), the final pathology results were consistent with preoperative status, and both were diagnosed with tubulovillous adenoma accompanied by dysplasia (Figure 1). Consequently, of the 198 patients preoperatively diagnosed with malignant or benign periampullary tumors, 196 (98.99%) ultimately had PAC, for which surgery is essential.
Subgroup analysis
In the dysplasia subgroup (n=76), 74 patients (97.37%) initially diagnosed with dysplasia were finally proven to have malignant adenocarcinoma, and only the two remaining patients (2.63%) showed consistent postoperative and preoperative pathology results of dysplasia (Figure 2C). As for the inflammatory non-neoplastic lesion subgroup, the final pathology showed that all seven patients had PAC (Figure 2D).
Necessity for multiple preoperative biopsies
In the entire cohort (n=198), 191 patients underwent one preoperative endoscopic biopsy, and seven underwent two biopsies. At the first biopsy (n=198), 111 patients (56.06%) were diagnosed with PAC, but the postoperative pathology results showed there were 196 PAC patients in the whole cohort; thus, the sensitivity of one biopsy was 111/196 (56.63%) and the false-negative rate, also called the omission diagnostic rate, was 85/196 (43.37%) (Figure 3A). Furthermore, the seven patients with PAC diagnosed as having benign lesions at the first biopsy underwent a second biopsy, and 4/7 (57.14%) patients were finally diagnosed with PAC, which suggests the necessity for multiple preoperative biopsies (Figure 3B).
Tumor markers, lymph nodes and TNM status
We also analyzed the changes in tumor markers in patients with available data (Table 2). Postoperatively, of the 81 patients diagnosed with PAC, CA-199 levels were elevated in 36/80 (45%) patients; 4/37 (10.81%) patients showed high levels of CA-125, and CEA levels were elevated in 5/81 (6.17%) patients. Both CA-125 and CEA levels were normal in the two patients with a final pathology of dysplasia, but one patient showed a high CA-199 level (>700 U/mL).
Table 2
| Tumor markers | High, n (%) | Normal, n (%) | Total, n |
|---|---|---|---|
| CA19-9 | 36 (45.00) | 44 (55.00) | 80 |
| CA125 | 4 (10.81) | 33 (89.19) | 37 |
| CEA | 5 (6.17) | 76 (93.83) | 81 |
Note: all patients were diagnosed with cancer on the postoperative pathological biopsy. Normal ranges: CA19-9 0–37 U/mL; CA125 0–35 U/mL; CEA 0–5 ng/mL.
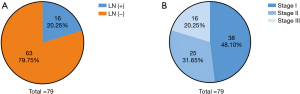
Furthermore, 16/79 (20.25%) patients preoperatively diagnosed with non-malignant lesions showed positive lymph nodes after surgery, suggesting that preoperative biopsy is unrelated to lymph node invasion (Figure 4A). Moreover, some patients preoperatively diagnosed with benign tumors may have metastatic malignancies. According to the American Joint Committee on Cancer (AJCC, 8th edition) cancer staging system, the final pathological diagnosis indicated 38 patients (48.10%) were stage I, 25 patients (31.65%) were stage II, and 16 patients (20.25%) were stage III among 79 patients with PAC with available lymph node status (Figure 4B).
Discussion
Accurate preoperative diagnosis is crucial for PAC patients, as the management strategy can be determined by the results of preoperative endoscopic biopsy (11). Patients preoperatively diagnosed with malignancy require PD, with its attendant high risk of severe complications such as infection, pancreatic leak, and bleeding (7,22). Patients with benign lesions can be treated conservatively (e.g., endoscopic papillectomy) to avoid severe complications (8-11). Consequently, an erroneous result of biopsy can potentially be disastrous for patients.
Endoscopic biopsy is the recommended method to obtain accurate preoperative pathological results, but the false-negative rate has been reported to be between 11.7% and 60% (15,17-21). There are several possible reasons for this finding. First, the special and complicated anatomic location around the ampulla of Vater increases the difficulty of obtaining samples, as the tumor could be in an unexposed area, making it difficult to detect (23). Second, it is difficult to exclude malignancy because the histopathology of dysplastic lesions is not homogeneous (24). Histomorphological studies support the hypothesis that invasive PAC arises from pre-existing mucosal lesions such as chronic inflammation or dysplasia (adenoma) (25). These premalignant lesions display cellular atypia that progresses from low-grade dysplasia (LGD) to high-grade dysplasia (HGD) and finally to invasive PAC. An adenoma can contain LGD, HGD, or malignancy at the same time (26). The initial finding of a benign pathology cannot exclude the presence of carcinoma in an unsampled part of the lesion (27). Furthermore, overlying mucosal lesions such as dysplasia may disguise deeper located carcinomas; therefore, the sampling of superficial lesions cannot rule out the possibility of deeper cancer (20). In addition, tumor heterogeneity is one of the hallmarks of cancer and the main cause of drug resistance, leading to therapeutic failure (28).
Our results verified that endoscopic biopsy might not be as reliable as postoperative pathological results because in 85/196 (43.37%) patients malignancy was missed in the first preoperative biopsy. Of the 198 patients diagnosed with malignant or benign periampullary tumors on preoperative endoscopic biopsy, 196 (98.99%) were ultimately diagnosed with PAC, which shows a high risk of periampullary tumor malignancy. As for the subgroups, 74 patients (97.37%) in the dysplasia subgroup (n=76) and all 7 patients (100%) in the inflammation subgroup diagnosed with benign tumors on initial biopsy were proven to have malignant PAC. Therefore, we assume that periampullary tumors are largely malignant and require PD as curative treatment.
Based on the difficulty of differentiating between benign and malignant tumors before surgery and the decline in complication morbidity and mortality rates over the past few years following PD in oncology centers, PD remains the treatment of choice for periampullary dysplasia (adenoma) in patients who can tolerate it (23,25,29). Thus, in patients with dysplasia (adenoma) of the periampullary papilla, especially those with moderate to HGD, aggressive management with PD should be offered following the exclusion of high-risk patients. In recent years, there are emerging possible adjuvant strategies to prolong postoperative survival time. Targeting the YAP-autophagy circuit and the FOXM1-miR-552-DACH1/PCDH10/SMAD4 axis may offer new opportunities for therapeutic intervention against PAC (30,31). In addition, leflunomide plus gemcitabine has demonstrated inhibition of the growth of pancreatic ductal adenocarcinoma (32).
Villous and tubulovillous adenomas are the most common benign lesions of the papilla of Vater (23). They are considered premalignant lesions and carry a higher risk of malignant transformation (33). P53 mutation and Ki-67 high proliferative activity play important roles in the histogenesis of invasive adenocarcinoma (34,35). Malignant transformation frequently occurs in adenomas at the time of diagnosis (36), so it is essential to rule out the presence of cancer in these lesions. Despite progress in imaging and endoscopy, the percentage of preoperatively missed malignancies remains high. Based on our data, 18/19 (94.74%) patients preoperatively diagnosed with tubulovillous adenomas were finally diagnosed with PAC after surgery.
Consequently, it is important to determine how to increase the accuracy of preoperative biopsies. In our study, seven PAC patients were diagnosed with benign tumors at first biopsy, but a second biopsy diagnosed four patients (57.14%) with cancer. Therefore, we recommend multiple biopsies to increase the accuracy. Moreover, limited diagnostic accuracy may result from a small number and shallow biopsy samples (20,27). EUS-guided tissue sampling is expected to show higher diagnostic accuracy and sensitivity but is less widespread in facilities (37). Further prospective studies should determine whether endoscopic biopsy using more and deeper samples improves the preoperative diagnostic accuracy for periampullary tumors. A strategy of obtaining four or more biopsy samples excluding the orifice area is recommended to increase diagnostic accuracy and avoid complications of endoscopic biopsy, such as pancreatitis (27). Tumor cell is a known indicator of disease progression in pancreatic cancer, which can be shed from primary lesions such as intraductal papillary-mucinous neoplasia and pancreatic intraepithelial lesions early in pancreatic ductal adenocarcinoma development (38).
Our study had some limitations. First, the number of patient samples was limited, and a large sample study is needed for verification. Second, this study was a retrospective analysis, which is likely have some deviations in the results, and needs to be further confirmed by multicenter clinical trials. Third, EUS-guided tissue sampling was not performed in this study.
Taken together, we can safely draw the following conclusion: a single preoperative endoscopic biopsy has a high false-negative rate, so multiple sites and multiple biopsies taken from greater depth are recommended to increase biopsy accuracy; patients preoperatively diagnosed with dysplasia, especially higher grade dysplasia or tubulovillous adenomas, have a high risk of misdiagnosis or undergoing malignant transformation, so should undergo PD as a curative treatment.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 82160578); the Natural Science Foundation of Jiangxi Province (grant Nos. 20212BCJ23024 and 20202BAB216029); the Health Department of Jiangxi Province (grant No. 20198020); and the Education Department of Jiangxi Province (grant No. GJJ190019).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-412/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-412/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-412/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Second Affiliated Hospital of Nanchang University (No. 2013049). Since this study was retrospective and did not involve personal privacy, informed consent of previous patients could not be obtained objectively.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hester CA, Dogeas E, Augustine MM, et al. Incidence and comparative outcomes of periampullary cancer: A population-based analysis demonstrating improved outcomes and increased use of adjuvant therapy from 2004 to 2012. J Surg Oncol 2019;119:303-17. [Crossref] [PubMed]
- Hugenschmidt H, Labori KJ, Brunborg C, et al. Circulating Tumor Cells are an Independent Predictor of Shorter Survival in Patients Undergoing Resection for Pancreatic and Periampullary Adenocarcinoma. Ann Surg 2020;271:549-58. [Crossref] [PubMed]
- Romiti A, Barucca V, Zullo A, et al. Tumors of ampulla of Vater: A case series and review of chemotherapy options. World J Gastrointest Oncol 2012;4:60-7. [Crossref] [PubMed]
- Kim JH, Jeong JH, Ryoo BY, et al. Adjuvant Chemotherapy for Resected Ampulla of Vater Carcinoma: Retrospective Analysis of 646 Patients. Cancer Res Treat 2021;53:424-35. [Crossref] [PubMed]
- Key C, Meisner ALW. Cancers of the liver and biliary tract. Available online: https://seer.cancer.gov/archive/publications/survival/seer_survival_mono_lowres.pdf
- Ko AH, Nakakura EK. Adjuvant Therapy for Ampullary Cancer. JAMA Surg 2019;154:715. [Crossref] [PubMed]
- Gleeson EM, Pitt HA, Mackay TM, et al. Failure to Rescue After Pancreatoduodenectomy: A Transatlantic Analysis. Ann Surg 2021;274:459-66. [Crossref] [PubMed]
- Binmoeller KF, Boaventura S, Ramsperger K, et al. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc 1993;39:127-31. [Crossref] [PubMed]
- Desilets DJ, Dy RM, Ku PM, et al. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc 2001;54:202-8. [Crossref] [PubMed]
- Cheng CL, Sherman S, Fogel EL, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc 2004;60:757-64. [Crossref] [PubMed]
- Kim JH, Kim JH, Han JH, et al. Is endoscopic papillectomy safe for ampullary adenomas with high-grade dysplasia? Ann Surg Oncol 2009;16:2547-54. [Crossref] [PubMed]
- Al-Hawary MM, Kaza RK, Francis IR. Optimal Imaging Modalities for the Diagnosis and Staging of Periampullary Masses. Surg Oncol Clin N Am 2016;25:239-53. [Crossref] [PubMed]
- El Nakeeb A, El Sorogy M, Ezzat H, et al. Predictors of long-term survival after pancreaticoduodenectomy for peri-ampullary adenocarcinoma: A retrospective study of 5-year survivors. Hepatobiliary Pancreat Dis Int 2018;17:443-9. [Crossref] [PubMed]
- Rösch T, Hofrichter K, Frimberger E, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc 2004;60:390-6. [Crossref] [PubMed]
- Kimchi NA, Mindrul V, Broide E, et al. The contribution of endoscopy and biopsy to the diagnosis of periampullary tumors. Endoscopy 1998;30:538-43. [Crossref] [PubMed]
- Roberts KJ, McCulloch N, Sutcliffe R, et al. Endoscopic ultrasound assessment of lesions of the ampulla of Vater is of particular value in low-grade dysplasia. HPB (Oxford) 2013;15:18-23. [Crossref] [PubMed]
- Clary BM, Tyler DS, Dematos P, et al. Local ampullary resection with careful intraoperative frozen section evaluation for presumed benign ampullary neoplasms. Surgery 2000;127:628-33. [Crossref] [PubMed]
- Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc 2006;63:292-301. [Crossref] [PubMed]
- Yamaguchi K, Enjoji M, Kitamura K. Endoscopic biopsy has limited accuracy in diagnosis of ampullary tumors. Gastrointest Endosc 1990;36:588-92. [Crossref] [PubMed]
- Menzel J, Poremba C, Dietl KH, et al. Tumors of the papilla of Vater--inadequate diagnostic impact of endoscopic forceps biopsies taken prior to and following sphincterotomy. Ann Oncol 1999;10:1227-31. [Crossref] [PubMed]
- Beger HG, Treitschke F, Gansauge F, et al. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg 1999;134:526-32. [Crossref] [PubMed]
- Karim SAM, Abdulla KS, Abdulkarim QH, et al. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): Cross sectional study. Int J Surg 2018;52:383-7. [Crossref] [PubMed]
- Heidecke CD, Rosenberg R, Bauer M, et al. Impact of grade of dysplasia in villous adenomas of Vater's papilla. World J Surg 2002;26:709-14. [Crossref] [PubMed]
- Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol 1996;31:376-82. [Crossref] [PubMed]
- Baczako K, Büchler M, Beger HG, et al. Morphogenesis and possible precursor lesions of invasive carcinoma of the papilla of Vater: epithelial dysplasia and adenoma. Hum Pathol 1985;16:305-10. [Crossref] [PubMed]
- Treitschke F, Beger HG. Local resection of benign periampullary tumors. Ann Oncol 1999;10:212-4. [Crossref] [PubMed]
- Kim HN, Kim KM, Shin JU, et al. Prediction of carcinoma after resection in subjects with ampullary adenomas on endoscopic biopsy. J Clin Gastroenterol 2013;47:346-51. [Crossref] [PubMed]
- Zhang A, Miao K, Sun H, et al. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int J Biol Sci 2022;18:3019-33. [Crossref] [PubMed]
- Roder JD, Stein HJ, Böttcher KA, et al. Stented versus nonstented pancreaticojejunostomy after pancreatoduodenectomy: a prospective study. Ann Surg 1999;229:41-8. [Crossref] [PubMed]
- Wang X, Dou N, Wang J, et al. FOXM1-induced miR-552 expression contributes to pancreatic cancer progression by targeting multiple tumor suppressor genes. Int J Biol Sci 2021;17:915-25. [Crossref] [PubMed]
- Sun T, Peng H, Mao W, et al. Autophagy-mediated negative feedback attenuates the oncogenic activity of YAP in pancreatic cancer. Int J Biol Sci 2021;17:3634-45. [Crossref] [PubMed]
- Phan T, Nguyen VH, Buettner R, et al. Inhibition of de novo pyrimidine synthesis augments Gemcitabine induced growth inhibition in an immunocompetent model of pancreatic cancer. Int J Biol Sci 2021;17:2240-51. [Crossref] [PubMed]
- Tran TC, Vitale GC. Ampullary tumors: endoscopic versus operative management. Surg Innov 2004;11:255-63. [Crossref] [PubMed]
- Esposito I, Friess H, Büchler MW. Carcinogenesis of cancer of the papilla and ampulla: pathophysiological facts and molecular biological mechanisms. Langenbecks Arch Surg 2001;386:163-71. [Crossref] [PubMed]
- Takashima M, Ueki T, Nagai E, et al. Carcinoma of the ampulla of Vater associated with or without adenoma: a clinicopathologic analysis of 198 cases with reference to p53 and Ki-67 immunohistochemical expressions. Mod Pathol 2000;13:1300-7. [Crossref] [PubMed]
- Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol 1992;87:37-42. [PubMed]
- Poley JW, Campos S. Methods and outcome of the endoscopic treatment of ampullary tumors. Ther Adv Gastrointest Endosc 2020;13:2631774519899786. [Crossref] [PubMed]
- Feng Z, Wu J, Lu Y, et al. Circulating tumor cells in the early detection of human cancers. Int J Biol Sci 2022;18:3251-65. [Crossref] [PubMed]



