Bi-pedicle nipple-sparing mastectomy (modified Letterman technique) and TIGR mesh-assisted immediate implant reconstruction, in a patient with Cowden syndrome
Introduction
A 38-year-old patient with Cowden syndrome (1,2) referred by genetics services for high-risk surveillance and consideration of risk-reducing mastectomy. She had been on regular surveillance with annual mammography and clinical examination for a few years since her diagnosis. The patient had E-cup sized breasts with grade 4 ptosis; the sternal notch to nipple measured 29 and 12 cm from nipple to inframammary fold (IMF) and was keen to reduce to C-cup breasts. Preliminary discussions favored implant-based reconstruction following mastectomy due to bilateral procedure, the patient’s desire to resume work early and look after a young and active child.
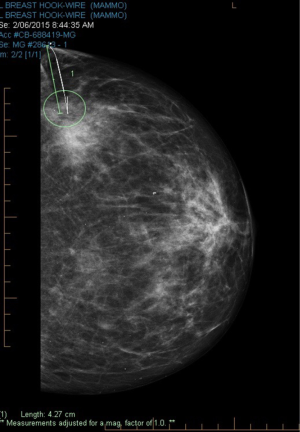
Routine annual surveillance mammogram identified a small focus of indeterminate micro-calcification in the upper outer left breast. Stereotactic core biopsy from this area showed atypical ductal hyperplasia (ADH-B3) on histology with recommendations of a hook-wire localized open breast biopsy for definitive pathology.
Breast multidisciplinary team meeting (MDT) reviewed the 7 mm area of indeterminate calcification at 3 o’clock, 80 mm from the left nipple, without any other concerning findings and normal contralateral right breast on diagnostic breast imaging. MDT discussions surrounding option of a one-stage procedure to include risk-reducing mastectomy vs. preliminary hook-wire localized breast biopsy for definitive histology, weighed towards initial diagnostic assessment.
In-depth discussions were undertaken with the patient following MDT recommendations but she was keen to avoid multiple procedures and recovery with additional time off work. Location of the micro-calcification and her desire to preserve both nipples, made a Wise-pattern skin-reducing mastectomy option, less favorable.
Operative technique
Patient was counseled pre-operatively and potential risks and complications outlined with clear instructions regarding postoperative care and management. Medical illustration photographs obtained with patient consent and detailed information leaflet about the procedure, including use of synthetic absorbable TIGR mesh (Novus Scientific®) provided to the patient.
Patient was warned of a higher than average risk of nipple necrosis/loss, based on the thin and narrow pedicles, with a low threshold for converting to a free-nipple graft, if intraoperative vascular compromise was evident.
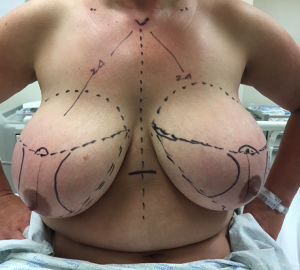
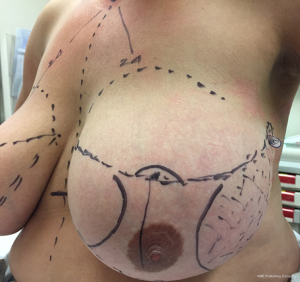
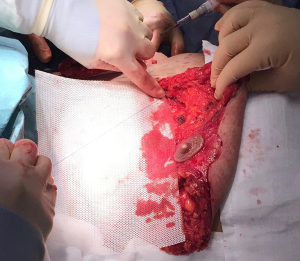
Preoperative skin markings were undertaken prior to hook-wire localization in the Radiology Suite (Figures 1,2). Lateral aspect of the left breast was marked to indicate desired location of hook-wire insertion (Figure 3) to minimize risk of a separate pinhole in the skin with potential for infection in an implant setting. Prophylactic intravenous antibiotic with 2 g of Cefazolin was given on induction and continued for 24 hours postoperatively. Paravertebral blocks by a specialist anesthetist were given sequentially during surgery to assist with postoperative analgesia.
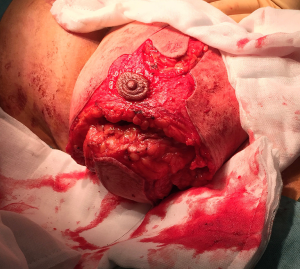
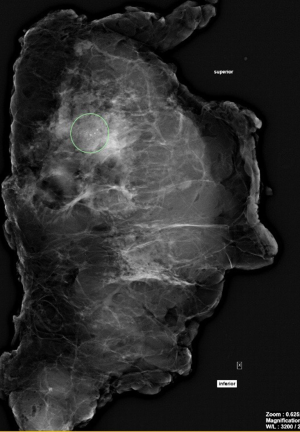
The mastectomy specimen with a paddle of skin overlying the area of interest including the hook-wire was sent for specimen X-ray to pathology (Figure 4). Specimen sectioned with hook-wire in-situ and imaged confirming targeted focus of calcification in the superior portion of slice 4 with good surrounding margin of normal tissue (Figure 5).
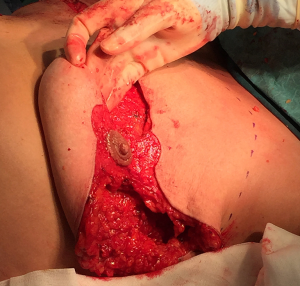
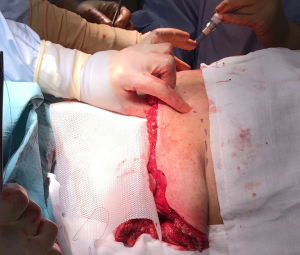
The mastectomy specimens were weighed intraoperatively with the right breast weighing 898 g and the left weighing 1,042 g. During the procedure, nipple viability was monitored regularly due to thin and narrows pedicles and was not compromised at any stage (Figure 6).
Pectoralis major muscle was gently lifted as per the preoperative skin markings, to create a pocket for the implant using diathermy throughout to ensure hemostasis.
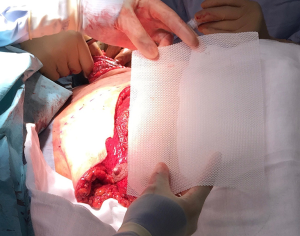
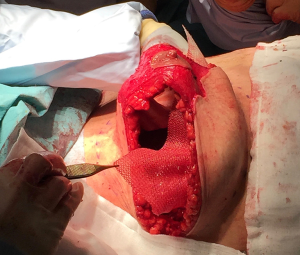
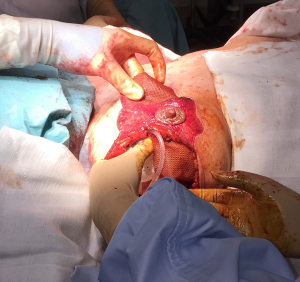
Medium size 15 cm × 20 cm TIGR matrix mesh (Novus Scientific®) was used to re-create the IMF (Figure 7) and secured with interrupted 3-0 PDS suture (Ethicon, J&J®) (Figures 8,9). This synthetic absorbable mesh made from co-polymer of glycolide, lactide and trimethylene carbonate, is easier to handle, produces lesser seroma with need for only one drain per side, compared to standard ADM in the author’s experience. This particular sized mesh offers adequate cover for the lower 1/3 of medium to large sized implants (Figure 10).
Temporary sizer used to estimate implant size and confirm closure of skin wounds without tension (Figure 11). Anatomical moderate height, moderate projection 560 cc Natrelle cohesive gel, textured silicone implants (Allergan, California®, USA) were used on each side, allowing tension-free closure of skin wound. Pectoral muscle was sutured to the TIGR mesh with interrupted 3-0 PDS once the implant was in a satisfactory position.
A single No.15 low-suction blake drain was placed under the skin flap and brought out via a separate incision in the lower axilla and secured with drain stitch on each side. Oral antibiotic was continued for the duration of the drains, which were removed on day 7 once the seroma output reduced to less than 30 mL/day.
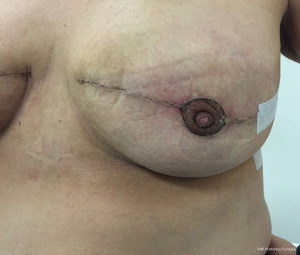
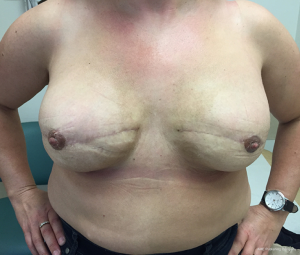
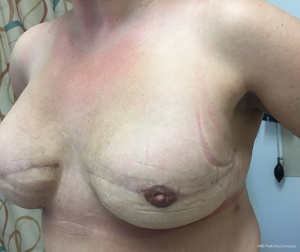
The patient was discharged home 2 days after surgery and closely monitored by the Breast Care Nurse. On week 3, patient developed a minor wound infection at the edge of the left nipple with moderate growth of serratia liquefaciens and corynebacterium species on culture (Figure 12). Course of oral ciprofloxacin antibiotic ensured salvage of the underlying implant. High level of patient satisfaction with cosmesis achieved and good functional return to work by week 5 (Figures 13-15).

Histology of the left breast showed 16 mm area of intermediate-grade DCIS with micropapillary and cribriform features and clear of all margins >10 mm, without any microinvasion. Additional findings of flat epithelial atypia (FEA) and proliferative features including papillary apocrine change, pseudoangiomatous stromal hyperplasia (PASH) and columnar cell change or hyperplasia were identified. There was no evidence of in-situ or invasive malignancy on histology of the right breast. Postoperative breast MDM discussion felt the patient had been adequately treated without the need for adjuvant therapy. She remains under active surveillance with medical oncology and the breast unit in Christchurch.
Discussion
Bi-pedicle nipple-preserving mastectomy for gynaecomastia, a modification of the Letterman technique, has been previously described (3,4). Historically, larger breasted women were offered two-stage procedure of breast reduction followed by skin-sparing mastectomy with reconstruction to achieve a final smaller-sized breast in the setting of risk-reducing mastectomy (5). Safety of skin-sparing mastectomy with ADM-assisted immediate implant reconstruction in patients with small early breast cancers and those with previous breast reduction scars has also been established (5,6). Search of medical literature including PubMed, did not identify use of the modified Letterman approach in the immediate implant reconstruction setting following mastectomy.
Potential pitfalls of nipple loss or necrosis can be circumvented by careful patient selection, avoiding in smokers, diabetics or older patients, careful handling of skin flaps and the pedicle to avoid traction injury and ensuring closure of all wounds without tension. Intraoperatively the pedicle was thinned down to avoid ghosting effect, which could have potentially compromised vascular supply to the nipple. Special nipple dressings were left undisturbed for a week to minimize risk of wound contamination. The transparent Tegaderm dressing with a window allows visual monitoring of nipple viability by nursing staff in the immediate postoperative period while keeping the wound sealed.
Successful outcome in this case was possible due to coordinated teamwork in a multidisciplinary setting between the specialist breast radiologist, anatomical pathologist, infectious disease specialist, specialist anesthetist and an oncoplastic breast surgeon. Careful patient selection and education with multiple discussions, detailed information leaflets and close monitoring in the post-operative period by a dedicated breast care nurse is vital.
Acknowledgements
Dr. Shelley Boyd, Specialist Breast Radiologist, Canterbury Breast Care, Christchurch, New Zealand; Dr. Gavin Harris, Clinical Director, Anatomical Pathology; Lead Breast Pathologist, Canterbury Health Laboratories, Canterbury District Health Board, Christchurch, New Zealand; Dr. Trudy Ballantine, Specialist Anesthetist, Christchurch Hospitals, Canterbury District Health Board, Christchurch, New Zealand; Professor Bridget Robinson, Medical Oncologist, Christchurch Hospitals, Canterbury District Health Board, Christchurch, New Zealand; Maria Winter, Breast Care Nurse Specialist, Department of Surgery, Christchurch Hospital, Canterbury District Health Board, Christchurch, New Zealand; Dr. Simon Dalton, Infectious Disease Specialist, Christchurch Hospitals, Canterbury District Health Board, Christchurch, New Zealand; Dr. Kyle Kowlosky, Breast and Endocrine Fellow, Department of Surgery, Christchurch Hospitals, Canterbury District Health Board, Christchurch, New Zealand; Dr. Usha Shan, Oncoplastic Breast and General Surgeon, Northland District Health Board, Whangarei, New Zealand.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst 2013;105:1607-16. [Crossref] [PubMed]
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract 2010;8:6. [Crossref] [PubMed]
- Letterman G, Schurter M. Surgical correction of massive gynecomastia. Plast Reconstr Surg 1972;49:259-62. [Crossref] [PubMed]
- Vaughn CJ, Peled AW, Esserman LJ, et al. Feasibility of Performing Total Skin-Sparing Mastectomy in Patients With Prior Circumareolar Mastopexy or Reduction Mammoplasty Incisions. Ann Plast Surg 2013. [Epub ahead of print]. [Crossref] [PubMed]
- Ward CM, Khalid K. Surgical treatment of grade III gynaecomastia. Ann R Coll Surg Engl 1989;71:226-8. [PubMed]
- Spear SL, Hannan CM, Willey SC, et al. Nipple-sparing mastectomy. Plast Reconstr Surg 2009;123:1665-73. [Crossref] [PubMed]