Lymphatic mapping and preoperative imaging in the management of post-mastectomy lymphoedema
Introduction
Lymphoedema after mastectomy is well recognized with 21% of women developing the disease after defeating breast cancer (1). The specific pathological mechanisms as to how lymphoedema develops is not fully understood but it is understood that women requiring axillary lymph node dissection are 4 times as likely to develop symptoms compared to those who underwent sentinel lymph node biopsy (2) and those who proceed to undergo radiotherapy and certain chemotherapy treatments are also at greater risk (3,4).
The time after breast cancer diagnosis and treatment that patients may develop lymphoedema symptoms may be up to 24 months and beyond hence the need to be vigilant to recognize symptoms such as swelling, pain and tightness to take appropriate steps towards establishing a diagnosis in this high risk group (5). The lymphatic system of the breast is an important route for cancer spread (6) in addition to its normal function of draining lymph fluid and therefore the ability to appreciate its anatomy will help towards non-surgical and surgical management.
Imaging the lymphatic system in patients with this disease burden can be undertaken by various modalities such as lymphoscintigraphy, ultrasound, magnetic resonance lymphangiography (MRL) and indocyanine green (ICG)-enhanced lymphography; each with its own strengths and weaknesses. The current paper aims to highlight the more readily available of these: lymphoscintigraphy, ICG lymphangiography and immunofluorescence, MRL and computed tomographic lymphangiography. With the use of such techniques, the diagnosis and treatment of lymphoedema is assessed.
The axillary lymphatic system
The breast is drained by the intramammary lymphatic chain and the axillary lymphatic chain through multiple routes including superficial and deep lymphatic channels (6).
The axillary lymphatic system drains lymph using five main lymph node groups: pectoral, subscapular, humeral, central and apical. The pectoral group drains the mammary gland and the anterior thoracic wall; subscapular group drain the posterior thoracic wall and periscapular region; humeral lymph nodes are the main conduit for draining lymph from the upper arm and the central group drain the pectoral, subscapular and humeral lymph nodes. All of the above are subsequently drained by the apical lymph node (7).
Lymphatic capillaries drain lymph (compromised of large proteins, lipids and water) from distal tissues and are made of endothelial cells with a basement membrane. Collagen filaments adhered to local connective tissue confers strength to these capillaries. Lymph fills these capillaries due to a favourable oncotic pressure gradient, contraction of the lymphatic capillaries, extrinsic pressure and forward lymph flow. Lymph is prevented from flowing backwards by valves. Lymphatic capillaries drain into collecting vessels (afferent channels) that feed into lymph nodes where it moves onto efferent channels and into the venous system (8,9).
Pathophysiology of lymphoedema
Lymphoedema can be described as congenital or acquired. Congenital lymphoedema presents as a result of developmental defects of the lymphatic system, and is less common than acquired lymphoedema that is related to cancer, management of cancer (radiotherapy and surgery) or due to trauma or other inflammatory processes (10).
The development of lymphoedema is multifactorial and may involve complex mechanisms that are not yet fully understood. A suggested example such as disruption of this lymphatic apparatus by mastectomy and axillary lymph node clearance reduces the ability of the remaining lymphatics to remove lymph. Increasing accumulation of protein rich interstitial lymph fluid favours the accumulation of water and causes loss of the structural integrity of remaining lymphatic system through lymph vessel dilation and valve failure. Fibrosclerotic tissue and collagen is deposited and inflammation ensues. Skin becomes thickened and displays characteristic peau d’orange appearance. It is unclear why certain why lymphoedema does not develop on all women who undergo breast and axillary node surgery or why there is often a delay before the disease begins (9,11).
Symptoms of lymphoedema
Diagnosis of lymphoedema depends on the clinician being able to identify risk factors in patients susceptible to lymphoedema and symptoms of the disease. Increased body mass index (BMI) and extensive surgery or axillary lymph node dissection increases the risk of lymphoedema and chemotherapy and hypertension may confer increased risk in specific groups of women (12,13).
Patients will exhibit symptoms in the upper limb and possibly the breast with symptoms such as pain, restriction in arm movement and feeling of fullness. They may demonstrate signs of swelling and redness as well as peau d’orange appearance of the breast in later disease (5,14).
Lymphoedema has a psychological burden on patients as well. Even in patients with no lymphoedema disease, the availability of better education and information surrounding breast cancer means that patients who have a preoperative fear of lymphoedema, younger breast cancer patients and those who face an axillary lymph node dissection will have higher postoperative fear of lymphoedema (15). In those that suffer lymphoedema after breast cancer, physical changes due the disease can affect confidence and self-image, and promote self-consciousness. Furthermore, they may develop emotional burden secondary to disease burden as they are reminded of their life changing event and may not only exhibit anger, fear and sadness but also progress into depression as a consequence (15-18).
In the general medical community, there is sub-optimal diagnosis and support for lymphoedema even though patients are keen for better awareness from their healthcare professionals (19,20).
Lymphatic mapping and pre-operative imaging
Lymphatic mapping is undertaken to understand the how specific tissues are drained by the lymphatic system and to determine the layout of the lymphatic vessels and nodes through which this is achieved. This aids in confirming diagnosis and planning operative or non-operative management (21).
It relies on the basic principle that interstitial fluid (basis of lymph) drains into lymphatic vessels with the tissue. These capillaries form lymphatic collectors that have lymphatic muscle cells and lymph node contractions that propel lymph towards lymph nodes. This mechanism also has the detrimental effect of helping metastasize cancer (22).
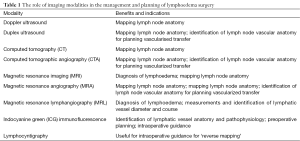
The use of these techniques can help to guide operative identification of both donor and recipient lymphatics (see Table 1, Figure 1).
Full table
Lymphoscintigraphy
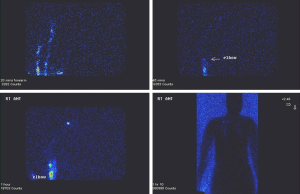
Lymphatic mapping can be undertaken by lymphoscintigraphy. This involves injecting a radiotracer into the hand (typically into the first and second webspace). The operator then qualitatively assesses various parameters such as how long the radiotracer takes to travel to the axilla, assessment of lymphatic capillaries, collecting ducts and nodes and also an assessment of severity and distribution of backflow through valves using Single-photon emission computed tomography (SPECT) or low dose computed tomography (CT). The operator can compare findings with previous ipsilateral studies or paired contralateral studies (see Figure 2). Further quantitative assessment can be undertaken through imaging the liver and assessing uptake (23).
Developments in the field, such as axillary reverse mapping (ARM) have been shown to identify further variations in lymphatic drainage of the upper arm. This begins with the same technique as standard lymphoscintigraphy as well as injecting methylene blue/IGC into several areas of the chest and back. Nodes which pooled methylene blue/IGC were of interest and dissected where as notes detected with a gamma probe as having taken up radiotracer injected into the upper extremity and can be carefully dissected around (selective axillary dissection) (24-26).
Several studies have shown that the use of ARM and selective axillary dissection can significantly reduce the incidence of lymphoedema after breast surgery and also patient perceived lymphoedema (26-29) however the literature in not unanimous and some studies found it had no benefit in reducing lymphoedema (30).
Magnetic resonance lymphangiography (MRL)
Magnetic resonance imaging (MRI) has been previously known to be able to characterize lymphoedema (detecting honeycomb patterns between muscle and subcutis above the fascia and signal intensification for T2 weighted sequences) (31).
MRL with contrast enhancement has been found to be a non-invasive investigation for extremity lymphoedema that can rapidly and accurately visualize lymph flow disturbances in lymphatic vessels as well as whether these vessels were still viable and assess the health and functionality of lymph nodes. Its non-invasive advantage and its higher resolution confer inherent advantages over traditional investigations such as lymphoscintigraphy and standard MRI (32,33).
Patients with early disease and clinical suspicion of lymphoedema may benefit from solitary or serial non-invasive MRL as it is able to detect and monitor changes in the patients’ lymphatic system and accurately map dilation of lymphatic vessels in millimeters, with a view to helping plan surgical intervention if appropriate (34).
Indocyanine green (ICG) lymphography
ICG is a water-soluble fluorescent dye that has been used for several decades, mainly to map blood flow in the heart, liver and eye when administered intravenously. In assessment of the lymphatic system, ICG is injected subcutaneously where it binds to albumin and is taken up by the lymphatic system where it can be detected using near infra-red range camera system deep in the tissue (35-40).
The benefit of ICG lymphography is that it can detect early and less severe disease in a more non-invasive manner compared to lymphoscintigraphy (34,41,42). Specific techniques using this medium can help understand and identify the pathophysiology (such as lymph pump function failure) in order to plan appropriate treatment (36,38). Differing doses of ICG can be used to achieve satisfactory visualization of the lymphatic system without affecting flow patterns (39). In addition, ICG imaging can allow identification of lymphatics not draining certain regions, demonstrated on other imaging such as lymphoscintigraphy (so called, reverse mapping as shown in Figure 3).
Specific staging systems using ICG such as the arm dermal backflow stage have been correlated with established clinical staging classifications like Campisi, to help the clinician to establish when to start treatment. Unlike lymphoscintigraphy, the injected dye is not radioactive thus it is conducive to serial measurements with greater safety (37).
In addition, varying patterns of ICG demonstration of lymphatics can aid in interpreting lymphatic anatomy. The four main patterns are ‘linear’, ‘splash’ (see Figure 4), ‘stardust’ (see Figure 5), and ‘diffuse’. Initially, the splash-back pattern represents dermal collecting lymphatics, while a stardust pattern occurring later represents lymphatic pooling at open ends of dermal collecting lymphatics. In severe lymphoedema, ICG pooling represents a diffuse pattern.
Management of lymphoedema
The management of lymphoedema is complex and the moment to intervene should be considered carefully. Timely diagnosis aids the clinician in formulating an appropriate management plan.
Initial management with compression therapy, physiotherapy and skin care may progress towards surgical interventions such as lymphatic venous anastomosis and lymph node transfer as non-operative methods do not address the underlying cause (43,44).
Traditional surgical approaches that reduced limb volume by debulking and resection approaches such as cutolipofascectomy and total surface lymphangiectomy have been followed by microsurgical reconstructive techniques that aim to not only offer symptomatic relief, but also to treat the underlying cause. While there are many microsurgical reconstructive options, as presented below, these have variable results reported in the literature (45-55).
Lymphatico-venous anastomosis
Diagnosis of lymphoedema
Lymphoedema results as internal and external manifestations of abnormal lymph transport and lymphatic system insufficiency. Disruption to the lymphatic architecture as a consequence of breast cancer surgery reduces the ability of the body to remove excess water, proteins and other cell products thereby allowing accumulation in the extracellular spaces. Chronic oedema can lead to lipodermatosclerosis and ulceration of skin. Furthermore, there is increased risk of infection.
Upper limb lymphoedema can be classified into:
- (I) Stage 0—sub-clinical disease with impaired lymph transport but no oedema;
- (II) Stage I—accumulation of protein rich fluid that features pitting oedema. Oedema resolves with elevation;
- (III) Stage II—oedema is not reduced by arm elevation and is non-pitting;
- (IV) Stage III—oedema results in irreversible skin changes such as fibrosis and warty overgrowths.
The majority of cases are diagnosed through history and examination. Care should be taken to consider co-morbidities such as obesity and to stage the disease accurately as early disease responds best to reconstructive microsurgical treatment. Various imaging modalities can be undertaken to both aid diagnosis and to plan intervention.
Imaging for diagnosis and pre-operative identification of lymphatics
Microsurgery to treat lymphoedema confers positive results, especially in the early stages, with pre-operative diagnostic work up using lymphoscintigraphy. Lymphoscintigraphy can determine if oedema is of lymphatic origin and thus if microvascular lymphatic surgery is appropriate. Techniques such as lymphatic-venous anastomosis or the use of venous grafts have best outcomes with early appropriate intervention and patient compliance to adjuvant therapies (44,49,50).
ICG for the preoperative assessment and marking of lymphatics for lymphatic-venous anastomosis has also been shown to offer acceptable results with significant reduction of oedema in small series of patients. Further information can be obtained through the use of ultrasound to mark potential venules for anastomosis alongside ICG assessment of lymphatics (51).
Preoperative MRI in addition to other diagnostic imaging modalities prior to surgical intervention offers further data at low risk to the patient and can lead to a good outcome (54,55).
Disease burden should be alleviated through the use of non-operative methods in patients with later stage disease before microsurgical intervention. Patients should also have access to robust support network and close clinical monitoring to maintain results. Poor patient compliance may lead to suboptimal and unsatisfactory results (45).
Lymph node transfer
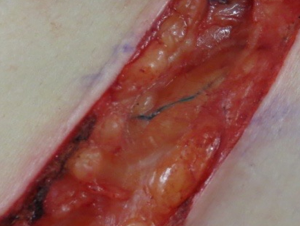
This technique relies on using lymphatic vessels as functional units and not merely channels to remove lymph. Much like a free flap, a vascularized lymph node with its associated vessels is removed from a donor site such as the groin to the graft site in the axilla or arm or forearm. Lymph nodes are understood to help the propulsion of lymph in their lymphatic vessels by contracting. Removal of the lymph node without its vascular supply alters the structure and functionality of the ‘unit’ and may impede lymph flow (56).
Use of lymph node transfer in the axilla has been shown in a series of 1,500 patients to normalize 40% of early stage lymphoedema and demonstrate clinical improvement in at least 95% of all stages of lymphoedema. This is thought to be due in part to production of vascular endothelial growth factor C by the newly transplanted lymph node promoting lymphoangiogenesis in addition to aiding lymph propulsion. The authors of this large study and other studies note the risk of subsequent donor site lymphoedema and other recognized complications such as difficulty monitoring the flap, infection and haematoma (57,58).
Pre-operative and intraoperative assessment of donor vascularized lymph nodes and recipient sites
Computed tomography (CT) angiography can demonstrate the location of vascularized lymph nodes for harvest in the groin to help plan the raising of lymph nodes supplied by the superficial circumflex iliac artery and vein or the superficial inferior epigastric artery and vein. Magnetic resonance angiography (MRA) has also been shown to delineate complex anatomy in the groin in preparation for vascularized lymph node harvest (59).
ICG can be used to assess the success of superficial groin free lymph node flap transfer to the elbow and wrist to treat post-mastectomy lymphedema. The anatomy of the flap was defined through cadaveric studies. Post anastomosis injection of ICG to the flap edge is drained into the donor vein and subsequently the recipient vein conferring statistically significant reduction in limb circumference when compared to patients who underwent conservative management only (60).
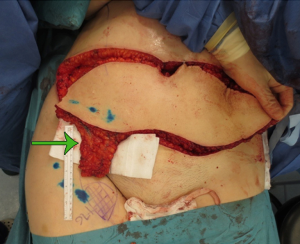
Reverse lymphatic mapping through the use of ICG has been used to reduce the risk of donor site lymphedema in vascularized lymph node transfer from the groin and axilla. For the groin, technetium is injected into the foot and drain to lymph nodes that drain the lower leg. Gamma probe identification of these nodes allows them to be avoided. Concurrently, ICG is injected to the lower abdomen, which, drains into lymph nodes in the groin that can be safely harvested. This is a similar case for the axilla, technetium injection into the hand identifies lymph nodes draining the upper limb that should be avoided and ICG injection to the chest and back will help identify vascularized lymph nodes that can be harvested relatively safely (61-63).
The right gastroepiploic lymph nodes have been harvested laparoscopically after preoperative assessment using CT angiography resulting in no donor site morbidity and satisfactory quality of life improvement in a series of ten patients (62,63).
Submental lymph node donor with an elliptical skin paddle along can be identified through the pencil Doppler ultrasound and the vasculature of supraclavicular lymph nodes can be identified intraoperatively through the use of ICG. Pre-operative ultrasound can help differentiate certain characteristics of potential lymph node basins (groin, supraclavicular or submental) as part of an initial feasibility assessment (63-66).
For upper limb lymphoedema, the recipient sites are the wrist, elbow and axilla. Preoperative assessment can be undertaken by ultrasound, CT or MRA. Intraoperative assessment can be undertaken by using ICG in this and other similar contexts (62-68).
Intraoperative identification of lymphatics
ICG can also be deployed to assess lymphatics intraoperatively using near-infrared cameras. The advantages are that it allows for quicker detection of lymphatics, concentrates the surgical field and optimizes theatre time. It has been shown to be safe by its routine use hepatic and ophthalmic assessments. However, disadvantages such as that the system is portable but can be unwieldy and not confer acceptable resolution for microsurgery (69).
The AccuVein vein visualization system can offer identification of venules to anastomose lymphatic vessels to by detecting near infra-red wavelengths and projects an image onto skin. The diameter of these subcutaneous venules can be as small as 0.5 mm (66-70).
The use of operative microscopes featuring integrated near infrared illumination system offers a solution to this problem and has been shown improve real time identification and dissection of lymphatics. It also aids the surgeon to achieve successful lymphatic venous anastomosis and has been shown to reduce disease burden in preliminary studies (67,68).
Lymphatic-lymphatic anastamosis
Often when lymphatic venous anastomosis is not possible when due to venous disease, it is possible to anastomose collecting lymphatics that have obstruction with unobstructed collecting lymph vessels that are located away from the area of pathology (61,63).
As with lymphatic venous anastomosis and lymph node transfer, treatment in patients with primary lymphoedema is more challenging and less often attempted due to developmental variations in lymphatic anatomy. Patients are investigated pre-operatively for this procedure through lymphoscintigraphy, magnetic resonance lymphography and ultrasound of the lymphatic system. However these investigations may not yield the necessary resolution to plan for lymphatic-lymphatic anastomosis (62). Ultimately, a range of preoperative imaging guides and intraoperative approaches may be needed to optimize identification and visualization of lymphatics (69-74).
Conclusions
Early detection and diagnosis of upper extremity lymphoedema in patients after mastectomy and axillary lymph node clearance is important in order to treat disease before it is too advanced to achieve favourable outcomes. Patients with disease refractory to conservative management can be efficiently assessed for diagnosis and surgical intervention using advanced lymphatic imaging techniques. The current paper has highlighted the more readily available of these: lymphoscintigraphy, ICG lymphangiography and immunofluorescence, MRL and computed tomographic lymphangiography in combination or individually. With such techniques, both diagnosis and treatment of lymphoedema has become more readily achieved, with lymphatico-venous and lymphatico-lymphatic anastomosis, and lymph node transfer now increasingly common undertakings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [PubMed]
- Mortimer P. Arm lymphoedema after breast cancer. Lancet Oncol 2013;14:442-3. [PubMed]
- Avraham T, Yan A, Zampell JC, et al. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am J Physiol Cell Physiol 2010;299:C589-605. [PubMed]
- Jung SY, Shin KH, Kim M, et al. Treatment factors affecting breast cancer-related lymphedema after systemic chemotherapy and radiotherapy in stage II/III breast cancer patients. Breast Cancer Res Treat 2014;148:91-8. [PubMed]
- Paskett ED. Symptoms: Lymphedema. Adv Exp Med Biol 2015;862:101-13. [PubMed]
- Wai CJ. Axillary anatomy and history. Curr Probl Cancer 2012;36:234-44. [PubMed]
- Macéa JR, Fregnani JH. Anatomy of the thoracic wall, axilla and breast. Int J Morphol 2006;24:691-704.
- Tanis PJ, Nieweg OE, Valdés Olmos RA, et al. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg 2001;192:399-409. [PubMed]
- Mayrovitz HN. The standard of care for lymphedema: current concepts and physiological considerations. Lymphat Res Biol 2009;7:101-8. [PubMed]
- Gasbarro V, Michelini S, Antignani PL, et al. The CEAP-L classification for lymphedemas of the limbs: the Italian experience. Int Angiol 2009;28:315-24. [PubMed]
- Stamatakos M, Stefanaki C, Kontzoglou K. Lymphedema and breast cancer: a review of the literature. Breast Cancer 2011;18:174-80. [PubMed]
- Togawa K, Ma H, Sullivan-Halley J, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res 2014;16:414. [PubMed]
- Sackey H, Magnuson A, Sandelin K, et al. Arm lymphoedema after axillary surgery in women with invasive breast cancer. Br J Surg 2014;101:390-7. [PubMed]
- Oliveri JM, Day JM, Alfano CM, et al. Arm/hand swelling and perceived functioning among breast cancer survivors 12 years post-diagnosis: CALGB 79804. J Cancer Surviv 2008;2:233-42. [PubMed]
- Taghian NR, Miller CL, Jammallo LS, et al. Lymphedema following breast cancer treatment and impact on quality of life: a review. Crit Rev Oncol Hematol 2014;92:227-34. [PubMed]
- Khan F, Amatya B, Pallant JF, et al. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast 2012;21:314-20. [PubMed]
- Vassard D, Olsen MH, Zinckernagel L, et al. Psychological consequences of lymphoedema associated with breast cancer: a prospective cohort study. Eur J Cancer 2010;46:3211-8. [PubMed]
- Chachaj A, Małyszczak K, Pyszel K, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology 2010;19:299-305. [PubMed]
- Barlow S, Dixey R, Todd J, et al. 'Abandoned by medicine'? A qualitative study of women's experiences with lymphoedema secondary to cancer, and the implications for care. Prim Health Care Res Dev 2014;15:452-63. [PubMed]
- Girgis A, Stacey F, Lee T, et al. Priorities for women with lymphoedema after treatment for breast cancer: population based cohort study. BMJ 2011;342:d3442. [PubMed]
- Blumgart EI, Uren RF, Nielsen PM, et al. Predicting lymphatic drainage patterns and primary tumour location in patients with breast cancer. Breast Cancer Res Treat 2011;130:699-705. [PubMed]
- Hansen KC, D'Alessandro A, Clement CC, et al. Lymph formation, composition and circulation: a proteomics perspective. Int Immunol 2015;27:219-27. [PubMed]
- Masia J, Pons G, Nardulli ML, et al. Lymphatic Imaging of the Breast: Evolving Technologies and the Future. In: Saba L, Rozen WM, Alonso-Burgos A, et al, editors. Imaging for Plastic Surgery. CRC Press, 2014:465-84.
- Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol 2007;14:1890-5. [PubMed]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [PubMed]
- Gennaro M, Maccauro M, Sigari C, et al. Selective axillary dissection after axillary reverse mapping to prevent breast-cancer-related lymphoedema. Eur J Surg Oncol 2013;39:1341-5. [PubMed]
- Yue T, Zhuang D, Zhou P, et al. A Prospective Study to Assess the Feasibility of Axillary Reverse Mapping and Evaluate Its Effect on Preventing Lymphedema in Breast Cancer Patients. Clin Breast Cancer 2015;15:301-6. [PubMed]
- Pasko JL, Garreau J, Carl A, et al. Axillary reverse lymphatic mapping reduces patient perceived incidence of lymphedema after axillary dissection in breast cancer. Am J Surg 2015;209:890-5. [PubMed]
- Boneti C, Badgwell B, Robertson Y, et al. Axillary reverse mapping (ARM): initial results of phase II trial in preventing lymphedema after lymphadenectomy. Minerva Ginecol 2012;64:421-30. [PubMed]
- Tausch C, Baege A, Dietrich D, et al. Can axillary reverse mapping avoid lymphedema in node positive breast cancer patients? Eur J Surg Oncol 2013;39:880-6. [PubMed]
- Duewell S, Hagspiel KD, Zuber J, et al. Swollen lower extremity: role of MR imaging. Radiology 1992;184:227-31. [PubMed]
- Liu NF, Lu Q, Jiang ZH, et al. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J Vasc Surg 2009;49:980-7. [PubMed]
- Arrivé L, Derhy S, El Mouhadi S, et al. Noncontrast Magnetic Resonance Lymphography. J Reconstr Microsurg 2016;32:80-6. [PubMed]
- Lohrmann C, Foeldi E, Langer M. MR imaging of the lymphatic system in patients with lipedema and lipo-lymphedema. Microvasc Res 2009;77:335-9. [PubMed]
- Akita S, Mitsukawa N, Kazama T, et al. Comparison of lymphoscintigraphy and indocyanine green lymphography for the diagnosis of extremity lymphoedema. J Plast Reconstr Aesthet Surg 2013;66:792-8. [PubMed]
- Yamamoto T, Narushima M, Yoshimatsu H, et al. Dynamic Indocyanine Green (ICG) lymphography for breast cancer-related arm lymphedema. Ann Plast Surg 2014;73:706-9. [PubMed]
- Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;128:941-7. [PubMed]
- Unno N, Nishiyama M, Suzuki M, et al. A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography. J Vasc Surg 2010;52:946-52. [PubMed]
- Aldrich MB, Davies-Venn C, Angermiller B, et al. Concentration of indocyanine green does not significantly influence lymphatic function as assessed by near-infrared imaging. Lymphat Res Biol 2012;10:20-4. [PubMed]
- Suami H, Chang DW, Yamada K, et al. Use of indocyanine green fluorescent lymphography for evaluating dynamic lymphatic status. Plast Reconstr Surg 2011;127:74e-76e. [PubMed]
- Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg 2008;36:230-6. [PubMed]
- Unno N, Inuzuka K, Suzuki M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg 2007;45:1016-21. [PubMed]
- Thomson M, Walker J. Collaborative lymphoedema management: developing a clinical protocol. Int J Palliat Nurs 2011;17:231-8. [PubMed]
- Boccardo F, Fulcheri E, Villa G, et al. Lymphatic microsurgery to treat lymphedema: techniques and indications for better results. Ann Plast Surg 2013;71:191-5. [PubMed]
- Campisi C, Bellini C, Campisi C, et al. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery 2010;30:256-60. [PubMed]
- Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol 2011;18:2500-5. [PubMed]
- Feldman S, Bansil H, Ascherman J, et al. Single Institution Experience with Lymphatic Microsurgical Preventive Healing Approach (LYMPHA) for the Primary Prevention of Lymphedema. Ann Surg Oncol 2015;22:3296-301. [PubMed]
- Campisi CC, Ryan M, Boccardo F, et al. LyMPHA and the prevention of lymphatic injuries: a rationale for early microsurgical intervention. J Reconstr Microsurg 2014;30:71-2. [PubMed]
- Vaqueiro M, Gloviczki P, Fisher J, et al. Lymphoscintigraphy in lymphedema: an aid to microsurgery. J Nucl Med 1986;27:1125-30. [PubMed]
- Campisi C, Eretta C, Pertile D, et al. Microsurgery for treatment of peripheral lymphedema: long-term outcome and future perspectives. Microsurgery 2007;27:333-8. [PubMed]
- Furukawa H, Osawa M, Saito A, et al. Microsurgical lymphaticovenous implantation targeting dermal lymphatic backflow using indocyanine green fluorescence lymphography in the treatment of postmastectomy lymphedema. Plast Reconstr Surg 2011;127:1804-11. [PubMed]
- Hara H, Mihara M, Seki Y, et al. Comparison of indocyanine green lymphographic findings with the conditions of collecting lymphatic vessels of limbs in patients with lymphedema. Plast Reconstr Surg 2013;132:1612-8. [PubMed]
- Mihara M, Hara H, Hayashi Y, et al. Upper-limb lymphedema treated aesthetically with lymphaticovenous anastomosis using indocyanine green lymphography and noncontact vein visualization. J Reconstr Microsurg 2012;28:327-32. [PubMed]
- Mihara M, Murai N, Hayashi Y, et al. Using indocyanine green fluorescent lymphography and lymphatic-venous anastomosis for cancer-related lymphedema. Ann Vasc Surg 2012;26:278.e1-6.
- Lohrmann C, Felmerer G, Foeldi E, et al. MR lymphangiography for the assessment of the lymphatic system in patients undergoing microsurgical reconstructions of lymphatic vessels. Microvasc Res 2008;76:42-5. [PubMed]
- Ito R, Suami H. Overview of lymph node transfer for lymphedema treatment. Plast Reconstr Surg 2014;134:548-56. [PubMed]
- Becker C, Vasile JV, Levine JL, et al. Microlymphatic surgery for the treatment of iatrogenic lymphedema. Clin Plast Surg 2012;39:385-98. [PubMed]
- Sulo E, Hartiala P, Viitanen T, et al. Risk of donor-site lymphatic vessel dysfunction after microvascular lymph node transfer. J Plast Reconstr Aesthet Surg 2015;68:551-8. [PubMed]
- Zhang H, Chen W, Mu L, et al. The distribution of lymph nodes and their nutrient vessels in the groin region: an anatomic study for design of the lymph node flap. Microsurgery 2014;34:558-61. [PubMed]
- Yamamoto T, Narushima M, Yoshimatsu H, et al. Minimally invasive lymphatic supermicrosurgery (MILS): indocyanine green lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann Plast Surg 2014;72:67-70. [PubMed]
- Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286-98. [PubMed]
- Ciudad P, Maruccia M, Socas J, et al. The laparoscopic right gastroepiploic lymph node flap transfer for upper and lower limb lymphedema: Technique and outcomes. Microsurgery 2015. [Epub ahead of print]. [PubMed]
- Raju A, Chang DW. Vascularized lymph node transfer for treatment of lymphedema: a comprehensive literature review. Ann Surg 2015;261:1013-23. [PubMed]
- Patel KM, Chu SY, Huang JJ, et al. Preplanning vascularized lymph node transfer with duplex ultrasonography: an evaluation of 3 donor sites. Plast Reconstr Surg Glob Open 2014;2:e193. [PubMed]
- Lee BB, Laredo J, Neville R. Current status of lymphatic reconstructive surgery for chronic lymphedema: it is still an uphill battle! Int J Angiol 2011;20:73-80. [PubMed]
- Mehrara BJ, Zampell JC, Suami H, et al. Surgical management of lymphedema: past, present, and future. Lymphat Res Biol 2011;9:159-67. [PubMed]
- Campisi C, Davini D, Bellini C, et al. Lymphatic microsurgery for the treatment of lymphedema. Microsurgery 2006;26:65-9. [PubMed]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013;46:1-11. [PubMed]
- Mikami T, Hosono M, Yabuki Y, et al. Classification of lymphoscintigraphy and relevance to surgical indication for lymphaticovenous anastomosis in upper limb lymphedema. Lymphology 2011;44:155-67. [PubMed]
- Mihara M, Hara H, Narushima M, et al. Lower limb lymphedema treated with lymphatico-venous anastomosis based on pre- and intraoperative icg lymphography and non-contact vein visualization: A case report. Microsurgery 2012;32:227-30. [PubMed]
- Yamamoto T, Yamamoto N, Numahata T, et al. Navigation lymphatic supermicrosurgery for the treatment of cancer-related peripheral lymphedema. Vasc Endovascular Surg 2014;48:139-43. [PubMed]
- Yamamoto T, Yamamoto N, Azuma S, et al. Near-infrared illumination system-integrated microscope for supermicrosurgical lymphaticovenular anastomosis. Microsurgery 2014;34:23-7. [PubMed]
- Ogata F, Narushima M, Mihara M, et al. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg 2007;59:180-4. [PubMed]
- Scaglioni MF, Suami H. Lymphatic anatomy of the inguinal region in aid of vascularized lymph node flap harvesting. J Plast Reconstr Aesthet Surg 2015;68:419-27. [PubMed]