Conservative mastectomies for breast cancer and risk-reducing surgery: the Memorial Sloan Kettering Cancer Center experience
Introduction
The surgical management of breast cancer has changed significantly in recent decades, from the disfiguring radical mastectomy, commonly performed until the mid 1970s, to breast-conserving surgery and sentinel lymph node biopsy, in which minimal breast tissue is removed and the morbidity associated with more extensive axillary surgery is avoided. There are, however, several circumstances in which mastectomy is still indicated, such as multicentric disease and inflammatory breast cancer (IBC), and circumstances in which breast radiation is contraindicated (such as pregnancy, previous breast-conserving therapy, and connective tissue diseases). In addition, some women diagnosed with breast cancer will choose mastectomy as their treatment of choice, even when breast conservation is possible, often undergoing a contralateral prophylactic mastectomy (CPM) at the same time (1). Risk-reducing surgery is also commonly performed in high-risk patients, such as those with a genetic predisposition to breast cancer and—in particular—those carrying a germline BRCA1 or BRCA2 mutation (2).
Oncoplastic surgery techniques enable surgeons to perform mastectomy with immediate or delayed reconstruction, preserving much of the skin envelope and sometimes the nipple-areola complex (NAC). Importantly, these dramatic changes toward more-conservative surgery have evolved without evidence of compromise to oncologic safety (3-17).
Increasing evidence that breast cancer is a systemic heterogenous disease requiring targeted systemic therapies has allowed for a change from the aggressive local therapies of the Halsted era to more conservative surgical approaches. The concept of performing mastectomy with preservation of skin and, if possible, the NAC, has developed gradually with the evolution of breast reconstruction, using either autologous tissue or implant-based techniques. “Subcutaneous mastectomy” followed by reconstruction was first reported in 1962 by Freeman, who described this procedure for patients with benign disease (18). The decline of the Halsted radical mastectomy in favor of the modified technique, which preserved muscle and some of the native breast skin, allowed for development of reconstructive surgery in breast cancer patients as well. The need for muscle cover to avoid implant exposure led to the use of tissue expanders to stretch the subpectoral pocket in preparation for a permanent implant.
The concept of a “skin-sparing mastectomy” was introduced by Toth and Lappert in 1991, with preservation of more of the native breast skin compared to the traditional modified radical mastectomy (19), resulting in improved cosmesis and a decrease in the need for contralateral symmetrization procedures (20). Initially, there was concern that preservation of more skin would result in increased rates of local recurrence; however, several studies have shown similar local recurrence rates to the modified radical mastectomy (3-9). It is now generally accepted that skin-sparing mastectomy is the standard mastectomy procedure, without an increased risk of disease recurrence and allowing for immediate breast reconstruction if desired.
Skin-sparing mastectomy with immediate reconstruction is now commonly performed; however, this procedure always involves removal of the NAC. Although several techniques exist for immediate or delayed nipple reconstruction, results are often unsatisfactory (21), and this has led to an increase in demand for the “nipple-sparing mastectomy (NSM)” procedure for both therapeutic and prophylactic surgery. The oncologic safety of NSM has been reported in many retrospective studies, with encouraging results (10-17), and as length of follow-up increases, this procedure is being accepted more and more as an option when specific criteria are met. In particular, it is often performed in the prophylactic setting, when such concerns regarding local recurrence do not apply.
The Memorial Sloan Kettering Cancer Center (MSKCC) experience
Indications for NSM
There are no widely accepted criteria that need to be fulfilled in order for NSM to be performed; however, several factors are considered for each potential candidate. Relative contraindications to NSM include smoking history, larger breast size, and ptosis. In breast cancer patients, those with skin or nipple involvement, larger (T3) tumors, central tumors close to the NAC, or blood stained nipple discharge are generally considered unsuitable candidates for NSM (22). All patients undergoing NSM meet with a breast surgeon and a plastic surgeon with a special interest in breast reconstruction preoperatively, to discuss the risks and benefits of NSM compared to skin-sparing mastectomy. Risks of NSM are discussed and documented, including inadequate blood supply leading to skin desquamation or necrosis, with the possible need for debridement or excision of the NAC. Concerns regarding oncologic safety, including lack of long-term data on local recurrence rates, are discussed with all patients, including those undergoing risk-reducing surgery.
Technical considerations
The most common incision performed for NSM at MSKCC is circumareolar, with lateral extension if required; however, the actual incision is decided on an individual case basis, following discussion between patient, breast surgeon, and plastic surgeon. Chen et al. have previously described the frequency, advantages, and disadvantages of different incisions used for NSM at our institution (23). The plastic surgeon will usually recommend a specific incision and discuss it with the breast surgeon. Agreement is reached between both surgeons and confirmed again on the day of surgery. We find that the circumareolar approach, with lateral extension as required, allows adequate access to the axilla for sentinel lymph node biopsy and axillary lymph node dissection when required. Although the lateral infra-mammary fold incision may have advantages in terms of cosmesis, most will agree that axillary surgery is more challenging using this approach, particularly in a large breast and when axillary lymph node dissection is required. Several factors are considered in each case when planning incision, including breast size, scars from previous surgery (if any), need for axillary dissection, and patient preference.
As with skin-sparing mastectomy, the aim is to remove all glandular breast tissue and retaining only a thin layer of subdermal tissue behind the NAC (<3 mm). The retroareolar space is typically infiltrated with 10 mL of saline at the beginning of the case to help develop the tissue plane between the breast tissue and the NAC. In all therapeutic cases, a section of retroareolar tissue (“nipple-margin”) is removed and examined either by intraoperative frozen section or routine permanent section as appropriate.
Review of NSMs performed at MSKCC
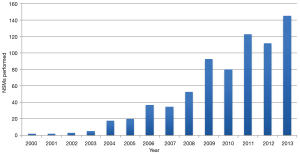
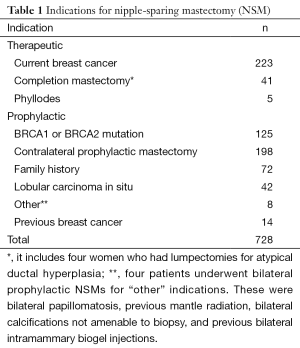
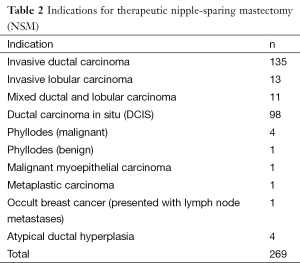
We recently reviewed our experience with NSM at this institution. Although skin-sparing mastectomy is by far the most common type of mastectomy performed, the number of patients undergoing NSM has significantly increased, as shown in Figure 1. During the period between 2000 and 2013, 728 NSMs were performed in 413 patients, 315 of whom underwent bilateral procedures (630 NSMs) and 98 of whom underwent a unilateral procedure. There were 269 therapeutic NSMs performed in 261 patients (eight patients had bilateral therapeutic NSMs). The remaining 152 patients underwent NSM for risk reduction. In addition, 176 patients undergoing therapeutic NSM also underwent a simultaneous CPM; therefore, the total number of patients undergoing a risk-reducing procedure was 328 (459 NSMs). The indications for NSM are shown in Tables 1,2.
Full table
Full table
Of 728 NSMs performed during this period, 177 were performed for risk reduction or treatment of breast cancer in 89 patients with a confirmed BRCA1 or BRCA2 mutation or a genetic variant of uncertain significance. We have recently reported our experience of NSM in this group of patients (24), and although follow-up is short at 27 months, there were no cases of local or regional recurrence, supporting the view that NSM is an acceptable option in patients with a BRCA1 or BRCA2 mutation.
Disease stage for patients undergoing therapeutic NSM
Following 269 therapeutic NSMs in 261 patients, the majority were confirmed to have pre-invasive or early-stage breast cancer (stage 0, n=81; stage 1, n=114; stage 2, n=51; stage 3, n=9; stage 4, n=0). The remaining patients had either phyllodes tumour (n=4) or had NSM for recurrent breast cancer (n=2) and were not assigned a disease stage.
Incidental diagnosis of ductal carcinoma in situ (DCIS) or invasive breast cancer following NSM
Invasive breast cancer was found unexpectedly in 26 patients. In eight cases, the patient was undergoing NSM for prophylaxis, and pathology revealed invasive ductal carcinoma (four cases) or invasive lobular carcinoma (four cases). The other 18 cases of unexpected invasive cancer were in patients undergoing therapeutic NSM for non-invasive disease who were subsequently upstaged; 17 of 98 women undergoing NSM for DCIS and one of four women undergoing NSM for atypical ductal hyperplasia. The type of invasive cancer was invasive ductal carcinoma in 17 cases, and mixed ductal and lobular in one case.
In addition, DCIS was found unexpectedly in 21 of 328 patients (22 breasts) undergoing prophylactic NSM (6.4%). This included a BRCA2 mutation carrier undergoing bilateral NSMs who was subsequently diagnosed with bilateral DCIS. One patient undergoing NSM for a benign phyllodes tumor was found to have DCIS. In total, 29 of 328 patients undergoing prophylactic NSM (8.8%) were diagnosed unexpectedly with either DCIS or invasive breast cancer.
Assessment of the “nipple margin”
Pathological assessment of the retroareolar “nipple margin” was positive for atypia or DCIS in 11 of 269 therapeutic cases. In 7 of these 11 cases, the NAC was excised, revealing residual DCIS in one case, atypia in another case, and five cases with no further disease in the excised specimen. Repeat nipple margin biopsies were performed in the remaining four cases, sometimes during the expander/implant exchange procedure. All of these were benign, allowing the NAC to be preserved. Of 459 prophylactic NSMs performed, four had a positive finding of DCIS in the retroareolar specimen. Three of these patients returned for excision of the NAC, and one patient, who was found to have only a very small focus of DCIS, decided against further surgery.
Follow-up
At median follow-up of 49 months (range, 0-149 months), 402 of 413 patients were alive with no evidence of disease. Four patients had died, one having developed regional and distant metastases 15 months after NSM for stage IIA disease, and another who had undergone nipple-sparing CPM developed metastatic disease from her initial stage IIIB breast cancer. One patient died of unknown cause, and another died of metastatic ovarian cancer. There was therefore one death attributed to metastatic breast cancer for which NSM had been performed.
Seven patients were alive with metastatic disease, two of whom had undergone nipple-sparing CPMs and developed metastases from the initial breast cancer. Five patients who had undergone therapeutic NSMs developed distant metastatic disease (all were initially diagnosed with either stage II or stage III disease). One of these patients was diagnosed simultaneously with both regional and bone metastases. No patient was diagnosed with local recurrence.
Breast reconstruction and complications
Immediate breast reconstruction was performed in almost all cases, with only 4 of 413 patients not undergoing reconstruction due to small breast size. The procedures performed were tissue expander/implant based (n=370), implant only (n=31), autologous flap (n=7), and fat injection only (n=1). Frequent use of ADM to cover the lower pole of the expander allows for greater initial fill volume, reducing the number of outpatient expansions. It also facilitates subsequent overexpansion, allowing for increased implant size if desired (23). The mean length of time between tissue expander insertion and exchange procedure was 169 days (median 143 days). Although 273 of 728 breasts (37.5%) had some degree of skin desquamation at follow-up, most of these were mild and fully resolved without intervention. Only 47 breasts (6.5%) developed skin necrosis requiring debridement. There were seven hematomas requiring evacuation (1%) and 31 wound infections (4.3%). Removal of expander/implant was required in 20 of 711 cases in which an expander/implant reconstruction was performed (2.8%).
Discussion
The evolution of conservative mastectomy techniques enables patients requiring mastectomy and patients undergoing risk-reducing surgery to benefit from advances in oncoplastic surgery, with improved cosmetic outcomes and reduced psychological impact. There are several forms of breast reconstruction available, using either the patient’s own tissue or prosthetic implants, or both. These procedures are made possible by skin-sparing and NSM techniques, which may allow the entire skin envelope and NAC to be preserved.
Data continue to show equivalence of these conservative techniques to the more traditional modified radical mastectomy in terms of local and regional recurrence rates (3-17). A recent database study from the United States of more than 20,000 women undergoing mastectomy for breast cancer from 1998 to 2007 showed a dramatic rise in the use of breast reconstruction (46% in 1998 to 63% in 2007). This change was predominantly due to a rise in the number of patients undergoing implant-based reconstruction, which is generally performed following a conservative mastectomy approach, with a decrease in the number of patients undergoing autologous tissue techniques during the same period (56% in 1998 to 25% in 2007) (25). Skin-sparing mastectomy has been the standard type performed at our institution for many years, and most patients undergo immediate implant-based reconstruction (26). The increased demand for nipple-sparing techniques, as discussed above, is further evidence of an expanding role for conservative mastectomies, in keeping with the increased demand for breast reconstruction in the United States (25).
Another significant change in breast surgery has been the increasing demand for CPM in breast cancer patients (27,28). We performed 198 NSMs in this context at our institution, including some patients with a previous history of mastectomy for breast cancer now presenting for delayed CPM. Patients will often choose bilateral mastectomy, even in the context of unilateral breast cancer amenable to breast-conserving surgery. Our surgeons spend many hours each week discussing the risks associated with bilateral surgery and the lack of survival benefit according to available evidence (29). Despite this, patients are often determined to pursue this course, and will sometimes change surgeons or hospitals in order to achieve this. It is essential that patients are making a fully informed decision regarding bilateral mastectomy and are aware of the risks, benefits, lack of impact on survival, and alternatives. Our cohort also includes patients with BRCA1 and BRCA2 mutations, and it is of course easier to justify CPM in these circumstances, particularly with recent evidence showing improved overall survival (30).
Suitability for NSM depends on several patient factors, including tumor size, skin involvement, and tumor proximity to the NAC. Although the number of patients requesting and being offered these procedures is increasing, it is certain that a group remains for whom conservative techniques are contraindicated. Patients with IBC require modified radical mastectomy following neoadjuvant chemotherapy, with subsequent post-mastectomy radiation therapy (PMRT) (31). By definition, these patients have skin involvement involving at least one-third of the breast, and skin biopsy may reveal tumor cells within dermal lymphatics. Immediate breast reconstruction in these patients is controversial and should generally be avoided (32). Although there have been reports of immediate reconstruction in IBC patients, local recurrence rates were high, particularly in the presence of positive mastectomy margins (33,34).
The requirement for PMRT, and the detrimental effect of this treatment to breast reconstruction of any form, is an important issue when considering the possibility of immediate breast reconstruction (35). Recent data from meta-analysis show that patients are likely to benefit from PMRT in the setting of limited axillary lymph node metastases (36), and it is likely that the use of radiation therapy in these circumstances will increase for this reason. In addition to the negative cosmetic effects of radiation therapy to a reconstructed breast, morbidities associated with reconstructive procedures can result in delays to commencement of PMRT, thereby possibly compromising oncologic treatment. Many patients will therefore forego immediate breast reconstruction in preference of a delayed procedure, and, in these circumstances, the benefit of the conservative mastectomy approach is less.
Almost all patients in this study underwent tissue expander/implant based reconstruction, and we have reported an acceptable complication rate, with 6.5% of patients developing skin necrosis requiring debridement. Only 31 patients (7.6%) underwent a single-stage implant-based reconstruction. Our preference is for a two-stage procedure for several reasons, as outlined in an earlier paper from our institution (23). Issues relating to nipple position asymmetry and implant asymmetry can be managed at the time of the replacement of the tissue expander with a permanent implant. Secondly, by limiting the volume of the tissue expander at the initial operation such that the skin envelope is expanded but not under tension, the risk of mastectomy skin flap and nipple-areola ischaemia is reduced. Finally, patients not infrequently request a size change, particularly if they are small breasted initially. Beginning the reconstructive process with a tissue expander allows the surgeon to customize the results to patient preference (23).
Although our experience with NSM is predominantly in patients with early-stage breast cancer (only 9 of 261 patients had stage III disease), there is emerging evidence that this technique is being used in patients with more advanced breast disease and with acceptable results. A recent report by Peled showed that of 753 patients undergoing NSM, 139 (18%) had locally advanced disease at diagnosis. The local recurrence rate was 5% at mean follow-up of 41 months (range, 4-111 months), and there were no recurrences in the preserved NAC (37). Although such reports are encouraging, it is important for patients to be informed that long-term data are lacking and that most existing data are based on patients with favorable disease characteristics. However, it is likely that we will continue to see an increase in the number of patients availing themselves of the NSM technique, particularly in the setting of risk-reduction surgery. The increasing use of conservative mastectomies represents further progress in the evolution of breast cancer surgery, lessening the psychological burden on those diagnosed with the disease and those undergoing risk-reducing surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Albornoz CR, Matros E, Lee CN, et al. Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer: The Role of Breast Reconstruction. Plast Reconstr Surg 2015;135:1518-26. [PubMed]
- Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:967-75. [PubMed]
- Carlson GW, Styblo TM, Lyles RH, et al. The use of skin sparing mastectomy in the treatment of breast cancer: The Emory experience. Surg Oncol 2003;12:265-9. [PubMed]
- Medina-Franco H, Vasconez LO, Fix RJ, et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg 2002;235:814-9. [PubMed]
- Meretoja TJ, Rasia S, von Smitten KA, et al. Late results of skin-sparing mastectomy followed by immediate breast reconstruction. Br J Surg 2007;94:1220-5. [PubMed]
- Kroll SS, Schusterman MA, Tadjalli HE, et al. Risk of recurrence after treatment of early breast cancer with skin-sparing mastectomy. Ann Surg Oncol 1997;4:193-7. [PubMed]
- Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence afterskin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol 1998;5:620-6. [PubMed]
- Kroll SS, Khoo A, Singletary SE, et al. Local recurrence risk after skin-sparing and conventional mastectomy: a 6-year follow-up. Plast Reconstr Surg 1999;104:421-5. [PubMed]
- Simmons RM, Fish SK, Gayle L, et al. Local and distant recurrence rates in skin-sparing mastectomies compared with non-skin-sparing mastectomies. Ann Surg Oncol 1999;6:676-81. [PubMed]
- Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol 2008;34:143-8. [PubMed]
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg 2009;249:461-8. [PubMed]
- Petit JY, Veronesi U, Rey P, et al. Nipple-sparing mastectomy: risk of nipple-areolar recurrences in a series of 579 cases. Breast Cancer Res Treat 2009;114:97-101. [PubMed]
- de Alcantara Filho P, Capko D, Barry JM, et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 2011;18:3117-22. [PubMed]
- Sakurai T, Zhang N, Suzuma T, et al. Long-term follow-up of nipple-sparing mastectomy without radiotherapy: a single center study at a Japanese institution. Med Oncol 2013;30:481. [PubMed]
- Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 2013;20:3218-22. [PubMed]
- Eisenberg RE, Chan JS, Swistel AJ, et al. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 2014;20:15-21. [PubMed]
- Adam H, Bygdeson M, de Boniface J. The oncological safety of nipple-sparing mastectomy - a Swedish matched cohort study. Eur J Surg Oncol 2014;40:1209-15. [PubMed]
- Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [PubMed]
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991;87:1048-53. [PubMed]
- Carlson GW, Bostwick J 3rd, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg 1997;225:570-5; discussion 575-8. [PubMed]
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg 2002;110:457-63; discussion 464-5. [PubMed]
- Garcia-Etienne CA, Cody Iii HS. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J 2009;15:440-9. [PubMed]
- Chen CM, Disa JJ, Sacchini V, et al. Nipple-sparing mastectomy and immediate tissue expander/implant breast reconstruction. Plast Reconstr Surg 2009;124:1772-80. [PubMed]
- Manning AT, Wood C, Eaton A, et al. Nipple-sparing mastectomy in patients with BRCA1/2 mutations and variants of uncertain significance. Br J Surg 2015;102:1354-9. [PubMed]
- Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol 2014;32:919-26. [PubMed]
- Manning AT, Cassella D, Ugras S, et al. Initial experience with an ambulatory extended recovery program for patients undergoing mastectomy. Cancer Res 2015:75:Abstr. P2-13-08.
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9. [PubMed]
- Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol 2009;27:1362-7. [PubMed]
- Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev 2010.CD002748. [PubMed]
- Heemskerk-Gerritsen BA, Rookus MA, Aalfs CM, et al. Improved overall survival after contralateral risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. Int J Cancer 2015;136:668-77. [PubMed]
- Rueth NM, Lin HY, Bedrosian I, et al. Underuse of trimodality treatment affects survival for patients with inflammatory breast cancer: an analysis of treatment and survival trends from the National Cancer Database. J Clin Oncol 2014;32:2018-24. [PubMed]
- Merajver SD, Iniesta MD, Sabel MS. Inflammatory Breast Cancer. In: Harris JR, Lippman ME, Morrow M, et al, editors. Diseases of the Breast. 4th edition. Philadelphia, Lippincott Williams & Wilkins, 2010:762-73.
- Slavin SA, Love SM, Goldwyn RM. Recurrent breast cancer following immediate reconstruction with myocutaneous flaps. Plast Reconstr Surg 1994;93:1191-204; discussion 1205-7. [PubMed]
- Chin PL, Andersen JS, Somlo G, et al. Esthetic reconstruction after mastectomy for inflammatory breast cancer: is it worthwhile? J Am Coll Surg 2000;190:304-9. [PubMed]
- Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 2011;127:15-22. [PubMed]
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [PubMed]
- Peled AW, Wang F, Foster RD, et al. Expanding the Indications for Total Skin-Sparing Mastectomy: Is It Safe for Patients with Locally Advanced Disease? Ann Surg Oncol 2015. [Epub ahead of print]. [PubMed]


