Undescended parathyroid adenomas as cause of persistent hyperparathyroidism
Introduction
Undescended parathyroid adenomas (UPA) are very uncommon cause of primary and secondary hyperparathyroidism (HPT). They are far more represented in series of parathyroid reoperations for recurrent or persistent HPT after a failed initial cervical exploration. If an inferior gland has not been identified at the initial operation in an orthotopic or low thymic location, an undescended parathyroid gland should be suspected.
Ectopic parathyroid glands can be located anywhere along the trajectory of their embryological descent. Inferior parathyroid glands are more suitable to descend to abnormal ectopic locations like the mediastinum or to descend incompletely and stand at the carotid sheath. This is likely related to their longer embryologic migration tract (1).
Historically, Hellstrom (2) reported that the term of “parathyroid” might be the reason why surgeons only explored the area around the thyroid gland. Weller (3) named the inferior parathyroid gland as parathymus, due to its vicinity to the thymus during embryologic development and its final position. This term is a reminder that surgeons should be aware of occasional need to explore a wider area of the thymus embryologic descent.
In the past preoperative invasive studies such as arteriography or selective venous sampling were often required to identify UPAs (4). Current development of noninvasive imaging techniques such as single photon emission computed tomography (SPECT) has improved the preoperative localization of UPAs and increased the surgical successful rate (5).
Materials and methods
We reviewed the database of patients evaluated and treated in our centre for persistent or recurrent primary and secondary HPT. Operative reports were reviewed to identify patients with an UPA. This was defined as a parathyroid adenoma found >1 cm above the upper pole of the thyroid gland in the vicinity of the common carotid artery bifurcation. Persistent or recurrent HPT was documented in all the patients by elevated serum calcium (>10.2 mg/dL) and PTH >55 pg/mL.
All the patients underwent localizing studies at our institution. Noninvasive studies consisted in ultrasonography, computed tomography, magnetic resonance imaging, technetium 99m sestamibi scintigraphy and single-photon emission computed tomography.
The operative strategy for each patient was determined on the basis of records from the initial exploration and the preoperative localization studies. A selective surgical approach was used in all cases. Intraoperative biopsy was performed to confirm the parathyroid nature of the lesion. Intraoperative PTH determination was used in two cases.
Results
Two patients with UPA were identified from a database of 598 parathyroidectomies for primary HPT (case 1) and 93 for secondary HPT (case 2) initially performed at our institution. A third case (case 3) was referred from another institution for persistent primary HPT-jaw tumor syndrome (JTS) with mutation in germ-line of HRPT2 (CDC73 type). No patient reported a previous family history. Clinical details of these three patients are shown in Table 1.

Full table
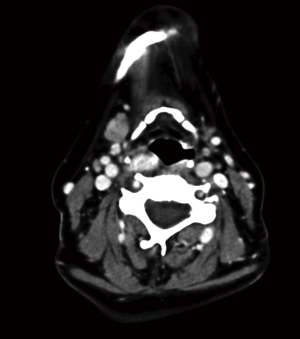
Case 1 underwent a bilateral neck exploration (BNE) for a thyroid nodule and primary HPT. A right thyroid lobectomy and left inferior parathyroidectomy were performed, with the pathological findings of benign thyroid nodule and parathyroid adenoma. Three normal parathyroid glands were identified. Hypercalcemia persisted and a both scintigraphy and a CT scan revealed a second undescended adenoma sitting on a fifth gland (Figure 1) that was confirmed at reoperation. Intraoperative PTH showed a curative descent from 239 to 69 pg/mL. The patient has remained normocalcemic for five years.
In case 2, a BNE was performed for secondary HPT with the identification and excision of three hyperplasic glands; the left inferior parathyroid gland was not found. A repeat parathyroid scintigraphy with an oblique projection disclosed a left UPA (Figure 2) that was excised at reoperation. An autotransplantation to the forearm was performed. He later received a kidney transplant.

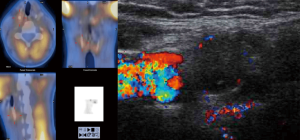
Case 3 was a young woman whose mother had been exposed to radiation in the Chernobyl disaster during her fourth month of pregnancy. As a child she underwent three thyroid and parathyroid explorations in her country of origin. A fourth procedure was performed in a referral Spanish unit and included a right inferior parathyroidectomy, reported as parathyroid carcinoma. Persistent HPT led to a fifth surgical procedure with excision of a cystic parathyroid gland and left thyroid lobectomy. At the time, a mutation in germ-line HRPT-2 was identified and was referred to us with the presumed diagnosis of parathyroid carcinoma with lymph node metastasis in the right lateral compartment II. A SPECT and a neck ultrasound (US) disclosed a hypoechoic nodule close to the carotid bifurcation (Figure 3). A right UPA consisting in two separate parathyroid adenomas was found and resected. Intraoperative PTH showed a curative descent from 180 to 21 pg/mL. The patient required calcium and vitamin supplementation for six months. At four years she is normocalcemic and her PTH is 20 pg/mL.
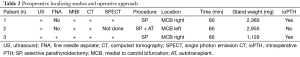
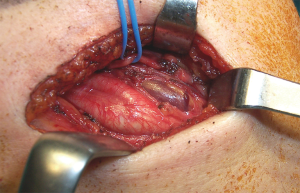
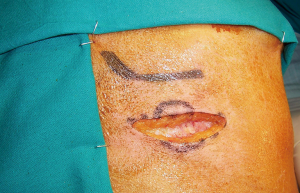
Preoperative localizing studies and operative approach are described in Table 2. A fine needle aspirate (FNA) was performed on case 3 to rule lymph nodes metastasis. All patients went on to have a successful focused procedure (Figures 4 and 5) through a high transverse lateral incision (Figure 6). In all cases the location of the UPAs was medial to the carotid bifurcation. The mean operative time was 76 minutes. Autotransplantation was performed in case 2, since three hyperplasic glands had been previously resected. The mean weight of UPAs was 1.6 grs. And two glands were excised in case 3.
Full table
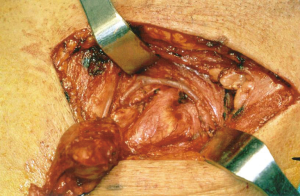
Discussion
In the fifth week of the embryogenesis the parathyroid glands arise from endodermal epithelial cells. The superior parathyroid glands derive from the fourth and the inferior glands from the third branchial pouch, the latter closely associated with the thymus. Therefore the inferior parathyroid glands have a longer route of embryologic descent and its final location at or around the lower pole of the thyroid lobe are variable. An inferior gland that fails to descend with the thymus remains at its embryologic origin close to the carotid bifurcation usually embedded in an ectopic thymic remnant (6,7).
From careful anatomic studies of 312 inferior parathyroid glands, Wang (8) concluded that UPAs occur in up to 2% of necks. In clinical series of previously unoperated primary cases, the incidence of UPAs is <1% (9). In the present report, only one UPA, case 1, was diagnosed (as second adenoma) in series of almost 600 parathyroidectomies; a 0.15% prevalence. UPAs were described by Simeone et al. (10) as an exceptional finding in five patients from a series of 3,000 patients (0.08%).
The incidence of UPAs in reoperative cases is higher, particularly in multiglandular HPT, as shown by cases reported here: one with a double adenoma and two with four gland diseases. In 1979, Edis et al. (1) reported seven UPAs in primary HPT patients: 1/414 found at initial operations (0.25%) and 6/27 at reoperation (22%). In a series of Fraker et al. (5) from 145 patients with persistent or recurrent HPT, nine (6.2%) had an UPA. A similar prevalence was reported by Shen et al. (11); from 102 patients who required reoperation, nine (8.8%) had an UPA. Billingsley et al. (12) reported 17 UPAs from series of 255 failed cervical explorations. These figures are similar to those reported in the present series.
UPAs localized by preoperative imaging studies are best removed by a focused approach through a high transverse lateral incision in the neck, one fingerbreadth below the mandible (Figure 6) (5,6). This avoids going through previously operated cervical structures and distorted surgical planes (13,14).
Two or more concordant preoperative imaging studies detect hyperfunctional parathyroid tissue in 60% to 70% of reoperative cases (15). The success rate of revision parathyroidectomy without preoperative localization is only 60%. Several observational studies, however, have reported success rate of 95% when a preoperative localization is possible (16,17). Localization studies identify multiple gland disease, in almost 20% of recurrent HPT (18).
In a recent study by Lee et al. (19) UPAs were identified in 16/5,241 patients who underwent parathyroidectomy at three referral centres (0.3% prevalence). Seven patients underwent selective approach (44%) while nine underwent bilateral exploration. Fourteen cases (87%) were primary operations thanks to a proper preoperative localization and two (12%) were revision cases. Cure rate was achieved in all patients and success rate of selective approach was best when there was a second concordant imaging study in addition to scintigraphy. This study differs from previously published reports in that most cases were first-time surgeries, suggesting that currently, the diagnosis of UPA is made before the initial neck exploration in specialized referral centres.
Studies on UPA in persistent multiglandular HPT are less common although recurrence is more likely in this setting. In the present review all three cases were related with some type of multiglandular disease. Secondary HPT involving an UPA is uncommon. Matsuoka et al. (20) presented a series parathyroidectomies for secondary HPT, where the prevalence of UPAs was 16/1,750 (0.9%). However, the rate of UPA in the reoperated patients is increased to 3.2%.
Case 3 is a good example of multiglandular genetic disease involving ectopic parathyroid tissue. HPT-JTS is a rare autosomal disease caused by inactivated germ-line mutations of HRPT-2 gene with subsequent loss of parafibromin expression. Some 20% to 30% of mutations develop spontaneously or may be radiation induced. Most patients with HRPT-2 mutation present with metachronous multiglandular involvement causing recurrence after selective parathyroidectomy (SP). HPT-JTS shows an increased risk of parathyroid carcinoma in 10-40% (21). Thus in these patients ectopic parathyroid adenomas, especially UPAs, can be misdiagnosed as metastatic lymph nodes. The more contemporary series of Mehta et al. (22) showed 20% of recurrent disease, 31% multiglandular involvement and 37.5% developed parathyroid carcinoma. Given the risk of malignancy and multiglandular involvement in this cohort, bilateral exploration is recommended and en bloc resection of parathyroid tumors suspicious for cancer, as well as life-long postoperatively follow-up.
Conclusions
UPAs were the cause of failed cervical exploration in 3 of 52 patients (5.6%) reoperated at our unit. All occurred in patients with multiple gland disease. Accurate preoperative localization of these lesions was possible with noninvasive studies and focused approach was performed successfully in all cases.
Although UPAs are exceptional as a cause of primary HPT (<1%), they are not uncommon among patients who require reoperation. Surgeons must take into account the possibility of an undescended gland both before the initial operation and, particularly, at revision surgery, when parathyroid adenomas cannot be found in orthotopic position.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Edis AJ, Purnell DC, van Heerden JA. The undescended “parathymus”. An occasional cause of failed neck exploration for hyperparathyroidism. Ann Surg 1979;190:64-8. [PubMed]
- Hellstrom J. The causes of unsuccessful or inadequate parathyroidectomy in hyperparathyroidism. Acta Chir Scand 1957;112:79-91. [PubMed]
- Weller GL. Development of the thyroid, parathyroid and thymus glands in man. Contrib Embryo 1933;24:93-139.
- Edis AJ, Sheedy PF, Beahrs OH, et al. Results of reoperation for hyperparathyroidism, with evaluation of preoperative localization studies. Surgery 1978;84:384-93. [PubMed]
- Fraker DL, Doppman JL, Shawker TH, et al. Undescended parathyroid adenoma: an important etiology for failed operations for primary hyperparathyroidism. World J Surg 1990;14:342-8. [PubMed]
- Bliss RD, Gauger PG, Delbridge LW. Surgeon's approach to the thyroid gland: surgical anatomy and the importance of technique. World J Surg 2000;24:891-7. [PubMed]
- Akerström G, Malmaeus J, Bergström R. Surgical anatomy of human parathyroid glands. Surgery 1984;95:14-21. [PubMed]
- Wang C. The anatomic basis of parathyroid surgery. Ann Surg 1976;183:271-5. [PubMed]
- Akerström G, Rudberg C, Grimelius L, et al. Causes of failed primary exploration and technical aspects of re-operation in primary hyperparathyroidism. World J Surg 1992;16:562-8; discussion 568-9. [PubMed]
- Simeone DM, Sandelin K, Thompson NW. Undescended superior parathyroid gland: a potential cause of failed cervical exploration for hyperparathyroidism. Surgery 1995;118:949-56. [PubMed]
- Shen W, Düren M, Morita E, et al. Reoperation for persistent or recurrent primary hyperparathyroidism. Arch Surg 1996;131:861-7; discussion 867-9. [PubMed]
- Billingsley KG, Fraker DL, Doppman JL, et al. Localization and operative management of undescended parathyroid adenomas in patients with persistent primary hyperparathyroidism. Surgery 1994;116:982-9; discussion 989-90. [PubMed]
- Chan TJ, Libutti SK, McCart JA, et al. Persistent primary hyperparathyroidism caused by adenomas identified in pharyngeal or adjacent structures. World J Surg 2003;27:675-9. [PubMed]
- Brennan MF, Norton JA. Reoperation for persistent and recurrent hyperparathyroidism. Ann Surg 1985;201:40-4. [PubMed]
- Feingold DL, Alexander HR, Chen CC, et al. Ultrasound and sestamibi scan as the only preoperative imaging tests in reoperation for parathyroid adenomas. Surgery 2000;128:1103-9; discussion 1109-10. [PubMed]
- Gough I. Reoperative parathyroid surgery: the importance of ectopic location and multigland disease. ANZ J Surg 2006;76:1048-50. [PubMed]
- Yen TW, Wang TS, Doffek KM, et al. Reoperative parathyroidectomy: an algorithm for imaging and monitoring of intraoperative parathyroid hormone levels that results in a successful focused approach. Surgery 2008;144:611-9; discussion 619-21. [PubMed]
- Jaskowiak N, Norton JA, Alexander HR, et al. A prospective trial evaluating a standard approach to reoperation for missed parathyroid adenoma. Ann Surg 1996;224:308-20; discussion 320-1. [PubMed]
- Lee JC, Mazeh H, Serpell J, et al. Adenomas of cervical maldescended parathyroid glands: pearls and pitfalls. ANZ J Surg 2012. [Epub ahead of print]. [PubMed]
- Matsuoka S, Tominaga Y, Uno N, et al. Surgical significance of undescended parathyroid gland in renal hyperparathyroidism. Surgery 2006;139:815-20. [PubMed]
- Iacobone M, Masi G, Barzon L, et al. Hyperparathyroidism-jaw tumor syndrome: a report of three large kindred. Langenbecks Arch Surg 2009;394:817-25. [PubMed]
- Mehta A, Patel D, Rosenberg A, et al. Hyperparathyroidism-jaw tumor syndrome: Results of operative management. Surgery 2014;156:1315-24; discussion 1324-5. [PubMed]


