Oncologic safety of conservative mastectomy in the therapeutic setting
Introduction
The term ‘conservative mastectomy’ has been formalised in recent years by Veronesi and colleagues from the European Institute of Oncology in Milan who accrued evidence in favour of nipple-preserving skin-sparing mastectomy procedures for early stage breast cancers of modest size and located away from the nipple-areola complex (NAC) (1). Nonetheless, the term conservative mastectomy has previously appeared in the literature but can be confusing prima facie due to the widespread use of the term conservative or conservation surgery for those procedures which aim to remove a localised tumour and a variable margin of normal surrounding breast tissue. In a sense, the term conservative mastectomy might be considered appropriate in the sense that this implies an extreme form of ‘breast conservation’ in which the wide excision is extended to include the whole breast parenchyma but leaves the skin envelope including the NAC intact. So in some respects this is a form of breast conservation, but at the same time is a type of mastectomy in terms of extirpation of all breast parenchyma. A distinction should be made between conservative mastectomy and other forms of nipple preserving mastectomy such as subcutaneous mastectomy which is often employed in the context of surgical prophylaxis (2). The latter aims are to preserve a thin sliver of breast tissue in order to ensure viability of the nipple-areolar complex. This operation is often undertaken in younger women with dense breast tissue which can be difficult to dissect off the under-surface of the NAC without compromise of vascular supply. By contrast, conservative mastectomy is a potentially curative procedure for established malignancy within the breast and aims for extirpation of all glandular tissue—it should be noted that these patients will not routinely receive adjuvant radiotherapy to the chest wall tissues which might otherwise treat any residual foci of breast tissue. It is imperative that conservative mastectomy is safe from an oncological perspective and not associated with higher rates of local recurrence compared with conventional or skin-sparing mastectomy without nipple preservation. Seminal breast conservation therapy trials have confirmed that preservation of the NAC as part of breast conserving surgery does not compromise overall survival and rates of local recurrence are acceptable when the remaining breast tissue is irradiated.
The aesthetic advantages of conservative mastectomy are well documented and recent data has emerged on psychological issues and other aspects of quality of life in women undergoing this type of mastectomy. However, the indications for conservative mastectomy remain to be defined and this procedure may not be appropriate for some women with larger breasts in whom reduction of the breast skin envelope is necessary. Preservation of the NAC as part of a skin-sparing mastectomy in patients for whom a mastectomy is otherwise indicated is of unproven safety and only practiced selectively for some patients with relatively small tumours located some distance from the NAC. Under these circumstances it might be reasonable to perform a conventional wide excision and oncoplastic glandular readjustment. The emergence of the conservative mastectomy has coincided with some important trends in surgical choice amongst breast cancer patients.
Breast conserving surgery
Breast conservation surgery has been established over the past 30 years as the preferred standard of surgical management for women with early stage breast cancer (3). Longer term follow-up data from several prospective randomized controlled trials have demonstrated survival equivalence for breast conservation therapy compared with radical or modified radical mastectomy (MRM/RM) (4-7). An update of National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 trial with 20 years follow-up confirms that post-operative irradiation improves local recurrence-free survival with similar distant disease-free and overall survival for MRM, wide local excision and radiotherapy or wide local excision alone (8). These findings suggest that residual cancer cells are a determinant of local failure but not distant disease. There is a finite rate of ipsilateral breast tumor recurrence (IBTR) for patients undergoing breast conservation therapy with recent estimates of between 3.5% and 6.5% at 10 years (9). Moreover, systemic therapies reduce rates of IBTR by approximately one-third and are halved with anti-HER2 directed therapies. Breast conservation surgery represents a balance between oncological mandates and cosmetic outcomes and aims to excise tumor with ‘negative’ margins and acceptable cosmesis. Rates of in breast recurrence are determined by negative margin status, but no direct relationship exists between margin width and IBTR (10). A consensus statement has decreed that an adequate margin exists when tumor is not touching ink and recommends this as the standard definition for invasive cancer (11). A negative margin does not imply absence of residual disease within remaining breast tissue but implies a residual burden of tumor sufficiently low to be controlled with adjuvant treatments (radiotherapy and chemo/hormonal therapies). Although histological examination of mastectomy specimens reveals that many tumors are multifocal with additional tumor foci beyond the index lesion, contemporary rates of IBTR after breast conserving therapy are very low. Local surgery does not completely eliminate residual disease burden with local recurrence determined by a combination of surgery, tumor biology, radiation and systemic therapies (9).
Changing trends in breast surgery
Increasing numbers of women are opting for ‘maximal surgery’ which implies removal not only of the ipsilateral breast but also the contralateral breast (even when otherwise suitable for breast conservation surgery for a unilateral breast cancer). In some instances contralateral prophylactic mastectomy (CPM) can be justified, such as for patients with carriage of BRCA gene mutations, but otherwise there is no widespread evidence to support CPM. In recent years there have been divergent trends—some women seek ‘maximal surgery’ whilst others prefer minimal surgical intervention with a desperate desire to preserve as much breast tissue as possible and avoid even unilateral mastectomy. Rates of CPM increased 150% between 1998 and 2003 (4% to 11%) and continue to do so with a doubling of CPM rates in the past 10 years (12). Furthermore, total mastectomy rates are decreasing in the USA but rates of breast conserving surgery have stabilized (13). With more women choosing ‘maximal surgery’, unilateral mastectomy has become a less commonly performed operation, but unilateral mastectomy rates are driving increased CPM rates. These trends are age dependent with dramatic increases in CPM amongst women <40 years of age (14). Nonetheless, increasing use of bilateral mastectomy is seen across all age groups, but is most pronounced for women <40 years. More women are choosing to undergo immediate breast reconstruction and requesting simultaneous CPM with breast reconstruction. The annual hazard rate for death from contralateral breast cancer has been decreasing since 1985 due to widespread use of adjuvant systemic treatment (15). Contemporaneous rates for development of contralateral breast cancer are about 0.2% per year and higher for those with BRCA mutations. Therefore rates of CPM are increasing, but paradoxically rates of contralateral breast cancer are decreasing.
This has to some extent been prompted by improvements in the availability and cosmetic outcomes of immediate breast reconstruction. Furthermore, many women chose implant-based reconstruction and it has been suggested that a desire to have matching breasts may be a driver for increased rates of CPM (simultaneous bilateral implant-based reconstruction) (16). Interestingly, patient satisfaction with bilateral implant-based reconstruction is higher than for unilateral reconstruction. The advent of nipple-sparing forms of mastectomy improves cosmetic outcome and may further increase rates of CPM. Long term outcomes from implant-based reconstruction are excellent and this desire to have matching breasts is relevant to increased rates of CPM. However, there may be surgical reasons for recommending mastectomy in more patients due to widespread use of pre-operative MRI for assessment of potential multifocality and confirmation of tumour size in patients who are otherwise considered suitable for conventional wide local excision ab initio. The indications for pre-operative MRI remain controversial and undoubtedly this modality of investigation is over-used and may have inadvertently led to increased rates of mastectomy—perhaps unnecessarily. Several studies have now assessed the effects of pre-operative MRI on either clinical (IBTR) or surgical (rates of re-operation) endpoints. In a retrospective study, Solin and colleagues examined rates of IBTR amongst a group of 756 patients, half of whom underwent breast conserving therapy based on conventional modes of staging whilst half had additional pre-operative MRI. Rates of IBTR were similar at 8 years for patients undergoing pre-operative MRI compared with those who did not (3% versus 4%) with no differences in contralateral breast cancer, breast cancer specific survival or overall survival (17). Likewise, a similar study of pre-operative MRI found that rates of IBTR were independent of whether this investigation was carried out or not (1.8% versus 2.5%) (18). MRI is highly sensitive for detection of cancer (lower specificity) and a meta-analysis found that additional tumor foci were identified in 20% of newly diagnosed breast cancer patients (16% in the ipsilateral and 4% in the contralateral breast) (19,20). Moreover, this actually led to a change in surgical therapy in between 8% and 33% of patients which was usually a change from breast conservation surgery to mastectomy. However, many of these additional foci were not confirmed on second biopsies and the incidence of additional foci is higher than the long term rates of IBTR. Indeed, some patients forewent additional biopsies of these MRI detected lesions because of perceived delays in final surgery (which was likely to be mastectomy with immediate breast reconstruction) (21). Furthermore, the randomised Comparative Effectiveness of Magnetic Resonance Imaging (COMICE) trial failed to show that use of pre-operative MRI (dedicated breast coil) in addition to imaging with mammography and ultrasound led to any reduction in rates of re-excision for those patients undergoing pre-operative MRI (22). Furthermore, as previously emphasized, there is no evidence for any reduction in rates of IBTR from routine pre-operative MRI examination which can detect additional tumor foci. This implies that many of these additional foci have no clinical significance and will be adequately treated with adjuvant therapies such as breast radiotherapy and chemo-hormonal therapies (9). It is therefore questionable whether there is an increasing need to perform total glandular excision on the basis of pre-operative MRI findings per se (1). Additional tumor foci, especially in different quadrants of the breast should be confirmed with biopsy—guided either by ultrasound or MRI if sonographically occult. A retrospective study involving more than 5,000 patients treated at the Mayo Clinic found that women who had pre-operative MRI were more likely to undergo mastectomy than those who did not have MRI (54% versus 36%; P<0.0001) (23). Though there is no causal relationship, this study did provide evidence for a link between increased usage of MRI and increased mastectomy rates.
Women often overestimate their risk of developing contralateral breast cancer but fail to appreciate that removal of the other breast will not improve overall survival which is usually determined by prognostic features of the ipsilateral cancer (4). Increased genetic testing (for BRCA-1 and BRCA-2) has strengthened the indications for bilateral prophylactic mastectomy and unilateral therapeutic mastectomy with CPM in patients with a strong family history of breast cancer. For these patients preservation of the NAC along with the entire breast skin envelope may be appropriate and should be aimed for in the context of risk-reducing procedures. However, this group constitutes only a small proportion of all breast cancer (5-10% at most) and is certainly not an explanation for increased rates of mastectomy in some units.
Oncological and technical aspects of nipple preservation
Oncological aspects
Although it is feasible to dissect the skin and subcutaneous tissues from the breast parenchyma without risk of leaving remnant breast tissue, this is not the case for the NAC; the main lactiferous ducts converge upon the nipple and breast tissue and are inextricably linked with the tissues of the nipple itself. The areola can be readily dissected off the underlying breast tissue but in younger women with dense breasts this can be technically challenging and sometimes a thin layer of breast tissue must be retained to ensure viability of the NAC. With a conservative mastectomy, excision of the retro-areolar tissue is a particularly important manoeuvre and a balance must be achieved between complete excision of the duct system and preservation of blood supply to the NAC. The ducts are usually cored out of the nipple, although micro-anatomical studies suggest that breast tissue within the nipple contains no terminal duct lobular units. There are two histological issues to consider with preservation of the NAC; firstly, leaving behind residual, but normal breast tissue and secondly the potential problem of leaving cancerous tissue/cells when surgery is performed as a therapeutic procedure for a known breast cancer. The proximal bundle of ducts can be examined pathologically using a frozen section specimen and the NAC removed when cancer cells are present. Sometimes mastectomy is the preferred surgical option on the basis of tumour size and not proximity to the NAC. There is no reason to suppose that tumors located away from the nipple would be associated with residual malignancy in the event of NAC preservation. In a retrospective pathological study of resected mastectomy specimens, malignant involvement of the nipple was found in 10.6% of cases. Moreover, cancer cells were found in the region of the nipple in only 6.7% of cases where the index lesion was a small (<2 cm) peripherally located tumor with no documented evidence of multi-focal lesions pre-operatively (24).
Despite these intuitive concerns about nipple sparing mastectomy for breast cancer, several groups have explored this procedure for smaller peripheral tumors situated more than 2 cm from the NAC. Enthusiasm for these approaches has been spurred on by reassuring reports about the oncological safety of skin-sparing mastectomy (25). Pioneering work from the European Institute of Oncology in Milan provided the foundations for the concept of conservative mastectomy (26). Between 2002 and 2007, just over 1,000 patients underwent nipple-sparing mastectomy for invasive ductal carcinoma (82%) or ductal carcinoma in situ (18%). All tumors were located away from the NAC at a minimum distance of 2 cm therefrom. Frozen section examination was undertaken in all patients at the time of surgery but there was a significant false negative rate associated with this procedure (8.6%). Moreover, 80% of patients received intra-operative radiotherapy (IORT) to the NAC with a single dose of 21 Gy from electron beams (ELIOT). In 20% of cases, radiotherapy was delayed due to ischaemic changes of the NAC intra-operatively (these patients subsequently received a fractioned dose of 16 Gy from a linear accelerator). There was a finite rate of partial (5.5%) and total (3.5%) nipple necrosis which necessitated removal of the nipple in 5% of cases. Patients with larger breasts were more susceptible to skin necrosis. An interim analysis at a median of 20 months follow-up (range, 1-69 months), revealed a very low rate of local recurrence (1.4%) and none of the relapsed cases involved the NAC directly. These very low rates of local recurrence with IORT prompt the question of whether acceptable rates of local recurrence for nipple-sparing mastectomy could be achieved without the use of radiotherapy. The Milan group have now published results from this cohort of patients at 50 months follow-up (27). A total of 11 (1.2%) patients have developed recurrence at the NAC [7 with DCIS (Paget’s disease); 4 with invasive carcinoma] with an overall survival of 96.4% at 5 years. Patients with local recurrence underwent complete excision of the NAC with no evidence of further recurrence at a median follow-up of 33 months. Interestingly, amongst the 8.6% of patients with a false negative frozen section, none have developed recurrence in the region of the NAC but half these cases were associated with local recurrence away from the NAC. Patients with widespread DCIS prompting mastectomy or invasive tumor with an extensive intraductal component were at higher risk of recurrence involving the NAC (DCIS is known to spread along ducts towards the nipple which may not be evident radiologically as microcalcification). Gerber and colleagues reported rates of local recurrence amongst a group of almost 300 patients for whom pre-operative investigations revealed a localised tumor more than 2 cm from the NAC without any extensive intraduct component (28). Patients underwent either skin-sparing mastectomy (51 patients), nipple-sparing mastectomy (61 patients) or conventional mastectomy (134 patients) with local recurrence rates of 10.4%, 11.7% and 11.5% respectively (P=0.974). Therefore no significant differences were noted for rates of local recurrence according to mastectomy type when patients were selected appropriately (small peripheral tumours without extensive DCIS and unlikely to require chest well radiotherapy). One should be wary about nipple-preserving procedure in patients for whom mastectomy would be indicated on surgical grounds, such as biopsy proven multifocal disease, larger primary tumours (>4 cm), location in more central parts of the breast and associated DCIS. In these circumstances, there is a risk of potential nipple involvement and rates of local recurrence may be increased in the future. Age, tumour size, nodal status and distance between tumour and NAC are crucial factors in selection of patients and minimising local recurrence. However, there is no clear association between tumor-NAC distance and rates of recurrence (29,30) with freedom from NAC recurrence reported in several studies where tumor-NAC distance was only 1 cm (27,31). Key questions to address for conservative mastectomy include the following:
- Is there an absolute upper size limit above which rates of local recurrence are unacceptable?
- What minimum distance between tumor and nipple should be stipulated?
- Should this distance be modulated by tumor size and is it best measured with MRI?
- Can conservative mastectomy be safely recommended for a small tumor (≤1 cm) which lies just outwith the NAC?
Paradoxically, these tumours can be managed with a central excision in large breasted patients, but no attempt is made to preserve the nipple which is excised en bloc with the tumor. In a comprehensive review of the available literature, Mallon and colleagues examined the incidence of occult nipple malignancy when nipple-sparing mastectomy was undertaken for primary breast cancer and found an overall incidence of 11.5% (32). Nipple involvement was statistically more likely (P<0.05) when associated with the following tumor characteristics (i) location <2 cm from the NAC; (ii) presence of nodal metastases; (iii) lymphovascular invasion; (iv) HER2 positivity; (v) negative hormone receptor status and (vi) size >5 cm. In addition, there was a greater chance of cancer within the NAC when tumors were multifocal and situated more centrally within the breast. The authors reported a nipple recurrence rate of 0.9% and concluded that nipple-sparing mastectomy for breast cancer was safe when patients were appropriately selected, namely with unifocal or well-circumscribed multifocal node negative, grade I or II, ER positive, HER2 negative tumors. Murthy and Chamberlain have further reviewed the evidence base for nipple sparing mastectomy and consider this a “reasonable alternative” for risk-reducing procedures and selected breast cancer patients. They emphasize the importance of pre-operative investigations and careful evaluation of MRI and mammographic features together with intra-operative frozen section examination. Moreover, standardization of pre-operative work up, intra-operative assessment and surgical technique is essential with clarification of radiotherapy delivery systems (intra-operative versus post-operative external beam) (33).
Technical aspects
Attention to surgical technique is especially important in the context of nipple-sparing mastectomy where careful and meticulous dissection deep to the NAC is essential to ensure both complete excision of ductal tissue with preservation of nipple vascularization. Skin-sparing mastectomy with sacrifice of the NAC is usually undertaken using a periareolar incision. This can be modified/extended if indicated to encompass skin overlying any tumor adjacent to the NAC and associated with skin tethering from involvement of the suspensory ligaments. By contrast, incisions must be adjusted accordingly when the NAC is preserved in order to retain a vascular supply from the adjacent mastectomy skin flaps via dermal vessels. Skin incisions can be placed around part of the NAC circumference with a lateral extension, but incisions placed remote from the NAC are preferred. These include the sub-mammary fold, a radial incision in the upper outer quadrant (which facilitates axillary surgery) or possibly a mid-axillary line incision when endoscopic-assistance is employed (34-38). Radial incisions have been popularised by the Milan group but may be less advantageous in the post-Z11 era when fewer patients undergo completion axillary lymph node dissection after a positive sentinel lymph node biopsy (the axilla should ideally be accessed through the breast incision and not a separate counter axillary incision) (39-41). Nonetheless, periareolar incisions are more likely to be associated with nipple necrosis and another alternative is the omega pexy incision (34). An interesting surgical manoeuvre is to dissect the retro-areolar tissue under local anaesthesia in advance of definitive surgery in order to ‘pre-condition’ the blood supply of the NAC by stimulating inflow of blood from the adjacent peripheral skin (42,43). The skin flaps for a conservative mastectomy are dissected in a similar manner to skin-sparing mastectomy with dissection along the cleavage or ‘oncological’ plane which lies between the subcutaneous fat layer and the superficial fascia of the breast. It is particularly important to maintain adequate thickness of the flaps in patients with larger breasts for whom the flaps will be proportionately longer and at higher risk of ischaemia. In some circumstances reduction of the breast skin envelope leads to malpositioning of the NAC which must then be sacrificed and subsequently reconstructed. Most surgeons prefer to preserve the pectoralis major fascia which assists with creation of an intact sub-pectoral pocket for implant-based reconstruction (44) (Figures 1-4). Another issue with conservative mastectomy is reduction of the skin envelope to achieve optimal shape of the breast with good ptosis—especially larger breasts. Hence, despite preservation of the NAC, some of the skin is sacrificed and no longer can it be claimed that there is ‘no disruption to the appearance of the breast’. Many surgeons do not preserve the NAC even when performing prophylactic mastectomy because of malpositioning in the reconstructed breast which may necessitate shift of the NAC at a later date. Likewise, immediate nipple reconstruction at the time of mastectomy and reconstruction for malignancy can lead to asymmetry with respect to the NAC.

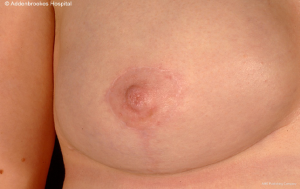
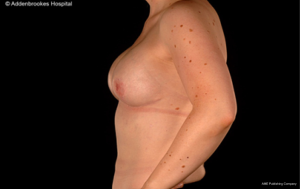
Immediate breast reconstruction after conservative mastectomy can be undertaken using either implant-based or flap-based techniques for small/moderate and larger sized breasts respectively. Patient satisfaction with bilateral implant-based reconstruction is higher than for unilateral reconstruction. Long-term outcomes from implant-based reconstruction are excellent and a desire to have matching breasts may be relevant to increased rates of CPM. However, there is evidence that unilateral implant-based reconstruction is worse than unilateral flap based reconstruction after 10 years in terms of maintenance of breast symmetry (16). Both expanders and fixed-volume implants can be used for reconstruction after conservative mastectomy and increasingly acellular dermal matrix is being used as an adjuvant material to reinforce the pocket and provide maximal implant coverage (45).
Cosmetic outcomes and quality of life
Cosmesis
Amongst the aforementioned group of 1,000 patients treated at the European Institute of Oncology in Milan, 414 were evaluated from an aesthetic perspective using a 10-point scale (1 worse; 10 best results). Overall score for both surgeons and patients was 8/10 with poorer scores relating to reduced sensitivity of the NAC rather than appearance of the reconstructed breast. Most patients underwent implant-based reconstruction with no significant differences in cosmetic outcome between expander and fixed-volume implants. Other groups have also reported favorable outcomes from conservative mastectomy in conjunction with implant-based immediate breast reconstruction (46,47). For patients undergoing autologous flap-based reconstruction, Gerber and colleagues reported notable decreases in cosmetic scores between the 5th and 9th years post-operatively whilst Denewer and Farouk observed excellent aesthetic outcomes in 82.9% of patients reconstructed with a modified extended latissimus dorsi muscular flap after conservative mastectomy (48). In addition, more than 90% of these patients experienced some nipple sensation post-operatively.
Psychological aspects
Using a specially developed patient questionnaire, researchers in Milan have examined the impact of conservative mastectomy on global health-related quality-of-life (49). Several key domains were evaluated including emotional status, anxiety levels, sexual functioning and body image amongst a group of well-educated women with an average age of 46 years. Women who underwent conservative mastectomy felt more comfortable viewing their own naked body and being seen by their partners compared with women who had reconstruction of the NAC after skin-sparing mastectomy. The sense of mutilation was significantly less, and cosmetic satisfaction significantly higher for conservative mastectomy patients who considered preservation of the NAC important in coming to terms with a cancer diagnosis and perception of a normal body image. Of note, these psychological benefits were not offset by an increased fear of recurrence due to retention of the NAC. A further analysis has revealed that a majority of women chose conservative mastectomy believing that nipple preservation will reduce psychological stress, enhance body image and improve overall satisfaction with results of breast surgery (50).
Conclusions
The procedure of ‘conservative mastectomy’ is appropriate for those patients who would otherwise be suitable for conventional wide excision with preservation of the NAC but who (for one reason or another) request mastectomy from personal choice. These patients are not recommended mastectomy from a surgical point of view and indications for conservative mastectomy remain to be defined. Where there is biopsy proven evidence of multicentricity which does not involve nor encroach upon the NAC radiologically, then conservative mastectomy may be indicated from a surgical perspective. There is currently no evidence that women should undergo ‘maximal’ surgery based on MRI findings of multicentric cancer. An important issue is post-mastectomy irradiation and demonstration of low rates of recurrence for NAC irradiation following nipple-preserving mastectomy begs the question of whether radiotherapy can be selectively omitted for this group of patients.
Further data are required to prove the oncological safety of conservative mastectomy and to define both selection criteria and the need for irradiation of the NAC. On the basis of evidence to date, it seems reasonable to exclude conservative mastectomy in those cases with evidence of extensive DCIS and multifocality—particularly when additional foci lie in the central zone of the breast. These questions demand further high quality studies before conservative mastectomy is widely adopted for localised cancers in patients without a confirmed BRCA gene mutation or strong family history of breast cancer. Limited conclusions can be drawn from small mono-institutional studies where much heterogeneity in practice exists (51). Rates of IBTR after breast conserving surgery have reduced dramatically in recent years and therefore conservative mastectomy has less potential for any oncological benefit in the absence of any overall survival gain. The main advantages of this procedure are likely to derive from its psychological and cosmetic benefits compared to oncoplastic breast conserving surgery. Nonetheless, “rigorous scientific scrutiny” (33) of this technique is mandatory to confirm oncological equivalence with skin-sparing mastectomy for breast cancer patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Veronesi U, Stafyla V, Petit JY, et al. Conservative mastectomy: extending the idea of breast conservation. Lancet Oncol 2012;13:e311-7. [PubMed]
- Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [PubMed]
- Breast Cancer Screening For Women Ages 40-49--NIH Consensus Statement. Medscape Womens Health 1997;2:1.
- Sarrazin D, Dewar JA, Arriagada R, et al. Conservative management of breast cancer. Br J Surg 1986;73:604-6. [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [PubMed]
- Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412-9. [PubMed]
- Benson JR. Long-term outcome of breast conserving surgery. Lancet Oncol 2012;13:331-3. [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer--bigger is not better. N Engl J Med 2012;367:79-82. [PubMed]
- Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg 2002;184:383-93. [PubMed]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014;32:1507-15. [PubMed]
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9. [PubMed]
- Tuttle TM, Abbott A, Arrington A, et al. The increasing use of prophylactic mastectomy in the prevention of breast cancer. Curr Oncol Rep 2010;12:16-21. [PubMed]
- Jin J. Women with breast cancer who opt for contralateral prophylactic mastectomy may overestimate future risk. JAMA 2013;310:1548. [PubMed]
- Nichols HB, Berrington de González A, Lacey JV Jr, et al. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol 2011;29:1564-9. [PubMed]
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 2009;124:345-53. [PubMed]
- Solin LJ, Orel SG, Hwang WT, et al. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol 2008;26:386-91. [PubMed]
- Hwang N, Schiller DE, Crystal P, et al. Magnetic resonance imaging in the planning of initial lumpectomy for invasive breast carcinoma: its effect on ipsilateral breast tumor recurrence after breast-conservation therapy. Ann Surg Oncol 2009;16:3000-9. [PubMed]
- Ciatto S, Bernardi D. Is preoperative staging with breast MRI causing overtreatment with mastectomies? Womens Health (Lond Engl) 2012;8:119-22. [PubMed]
- Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007;356:1295-303. [PubMed]
- Pengel KE, Loo CE, Teertstra HJ, et al. The impact of preoperative MRI on breast-conserving surgery of invasive cancer: a comparative cohort study. Breast Cancer Res Treat 2009;116:161-9. [PubMed]
- Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010;375:563-71. [PubMed]
- Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 2009;27:4082-8. [PubMed]
- Simmons RM, Brennan M, Christos P, et al. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol 2002;9:165-8. [PubMed]
- Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence afterskin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol 1998;5:620-6. [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat 2009;117:333-8. [PubMed]
- Veronesi P, Stafyla VK, Caldarella P. Conservative mastectomy: a promising technique. Rev Bras Mastologia 2012;21:57-60.
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg 2009;249:461-8. [PubMed]
- Brachtel EF, Rusby JE, Michaelson JS, et al. Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol 2009;27:4948-54. [PubMed]
- Loewen MJ, Jennings JA, Sherman SR, et al. Mammographic distance as a predictor of nipple-areola complex involvement in breast cancer. Am J Surg 2008;195:391-4; discussion 394-5. [PubMed]
- Margulies AG, Hochberg J, Kepple J, et al. Total skin-sparing mastectomy without preservation of the nipple-areola complex. Am J Surg 2005;190:907-12. [PubMed]
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013;131:969-84. [PubMed]
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J 2013;19:571-81. [PubMed]
- Regolo L, Ballardini B, Gallarotti E, et al. Nipple sparing mastectomy: an innovative skin incision for an alternative approach. Breast 2008;17:8-11. [PubMed]
- Crowe JP Jr, Kim JA, Yetman R, et al. Nipple-sparing mastectomy: technique and results of 54 procedures. Arch Surg 2004;139:148-50. [PubMed]
- Maxwell GP, Storm-Dickerson T, Whitworth P, et al. Advances in nipple-sparing mastectomy: oncological safety and incision selection. Aesthet Surg J 2011;31:310-9. [PubMed]
- Nakajima H, Fujiwara I, Mizuta N, et al. Clinical outcomes of video-assisted skin-sparing partial mastectomy for breast cancer and immediate reconstruction with latissimus dorsi muscle flap as breast-conserving therapy. World J Surg 2010;34:2197-203. [PubMed]
- Leff DR, Vashisht R, Yongue G, et al. Endoscopic breast surgery: where are we now and what might the future hold for video-assisted breast surgery? Breast Cancer Res Treat 2011;125:607-25. [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [PubMed]
- Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 2013;14:297-305. [PubMed]
- Benson JR. Management of breast-cancer patients with sentinel-node micrometastases. Lancet Oncol 2013;14:266-7. [PubMed]
- Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011;18:1665-70. [PubMed]
- Palmieri B, Baitchev G, Grappolini S, et al. Delayed nipple-sparing modified subcutaneous mastectomy: rationale and technique. Breast J 2005;11:173-8. [PubMed]
- Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg 2011;127:27-33. [PubMed]
- Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:28-41. [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L. Nipple-sparing mastectomy with immediate implant reconstruction: cosmetic outcomes and technical refinements. Plast Reconstr Surg 2010;126:1460-71. [PubMed]
- Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg 2009;62:586-90. [PubMed]
- Denewer A, Farouk O. Can nipple-sparing mastectomy and immediate breast reconstruction with modified extended latissimus dorsi muscular flap improve the cosmetic and functional outcome among patients with breast carcinoma? World J Surg 2007;31:1169-77. [PubMed]
- Didier F, Radice D, Gandini S, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat 2009;118:623-33. [PubMed]
- Didier F, Arnaboldi P, Gandini S, et al. Why do women accept to undergo a nipple sparing mastectomy or to reconstruct the nipple areola complex when nipple sparing mastectomy is not possible? Breast Cancer Res Treat 2012;132:1177-84. [PubMed]
- Garcia-Etienne CA, Cody Iii HS. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J 2009;15:440-9. [PubMed]



