Robotic transaxillary thyroid surgery
Introduction
Since the nineteenth century, when Kocher implemented the classical cervical thyroidectomy, little has changed in this procedure (1). When performed by experienced surgeons, the cervical approach is highly reliable and relatively short but unfortunately leaves a noticeable scar. Further advances in surgical instrumentation have introduced the minimally invasive thyroid surgery. The endoscopic thyroid surgery resulted in less morbidity and smaller surgical scars and developed into several different techniques (2). Nevertheless, the endoscopic cervical approach is surgically challenging since the neck is a very confined space to use CO2 insufflation, with PaCO2 elevation, subcutaneous emphysema and air embolism reported (3). The endoscopic approach can be applied today to a small group of patients.
The non-cervical, remote access approaches originally developed primarily due to cosmetic considerations—poor wound healing of certain ethnic groups and the aversion in the Asian culture to neck scars (4). Ikeda et al. in 2000 were the first to develop the transaxillary endoscopic approach to the thyroid (5).
With the introduction of the Da Vinci robot (Intuitive Surgical, Sunnyvale, CA, USA), some surgeons have recognized its potential advantages. The South Korean team from Seoul, led by Chung, pioneered the transaxillary approach to the thyroid gland in late 2007 (1,6). The robotic-assisted transaxillary thyroid surgery (RATS) approach was first described in North America by Kupersmith and Holsinger in 2011 (7). Since it was first introduced, more than 3,000 RATS procedures were performed in South Korea, and more than 6,000 worldwide (8). Among the other robot-assisted thyroidectomy (RT) approaches [facelift approach, bilateral-axillary breast approach (BABA)], the transaxillary became the most popular. The initial RATS was performed via two incisions (axillary and anterior chest wall), but later the modification using a single axillary incision was described (1,5).
Since the first report of RATS by the Seoul team, it has gained much popularity and interest in other parts of the world. Several groups have published their initial successful experience (9). However, since the conventional approach is safe, effective, and time-honored, some surgeons doubt the value of using robotic thyroid surgery and its clinical use (10). Robotic thyroidectomy, including RATS, remains controversial, especially in the west and the USA, where the FDA has revoked the approval on the use of robotic thyroidectomy and parathyroidectomy in 2011 (9).
Although several eligibility criteria to RATS were described, no standard selection criteria have been established (11). Absolute contra-indications are previous neck surgery or irradiation, retrosternal thyroid extension, and advanced thyroid disease (invasion of trachea, esophagus, distant metastases). Relative contra-indications are patient co-morbidities, age, obesity, very large goiters, well-differentiated carcinomas with a diameter larger than 2 cm, lateral neck metastases, and previous ipsilateral shoulder dysfunction (5,12,13).
Surgical technique
Creation of the working space (Figure 1)
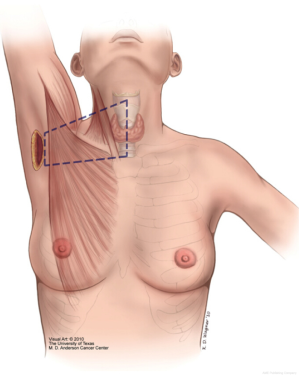
The axillary incision is defined in its inferior border by a horizontal line, from the sternal notch. The superior border—by an oblique line, is at a 60-degree angle from the thyroid notch. Some surgeons prefer to mark the incision while the patient is sitting, with the arms relaxed in a natural position, to verify it is camouflaged.
The use of endotracheal tube with laryngeal nerve monitoring is recommended. Following anesthesia, the patient's arm is placed in an extended position over the head, with a 90-degree flexion at the elbow. The arm should be carefully rotated and padded.
Following the axillary incision (5-6 cm), a dissection is performed in the subcutaneous plane, superficial to the pectoralis major muscle, to the clavicle. At the sternoclavicular joint, the sternal and clavicular heads of the sternocleidomastoid muscle, are identified. The dissection continues between these two heads to expose the strap muscles and deeper, the thyroid gland. Care should be taken during this step to avoid injury to the internal and external jugular veins. A this point, the retractor is inserted.
Docking of the robot (Figure 2)
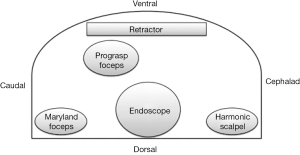
The Da Vinci cart is placed in the contra-lateral side. All three arms are inserted through the axillary incision (Prograsp forceps, harmonic shears and Maryland dissector), as well as the 30 degrees camera. The proper alignment of the robotic arms is crucial to avoid collision of the robotic arms inside the working space and the general success of the procedure.
Robotic thyroidectomy (console time)
The thyroidectomy is performed in the classical fashion: first, dissecting and safely transecting the superior thyroid vessels; second, the lobe is retracted medially to help identify the parathyroid glands and the recurrent laryngeal nerve (RLN). After ligating the inferior thyroid vessels and identifying the trachea, the lobe is carefully dissected from Berry’s ligament and extracted through the axillary incision. A drain is placed in the thyroid bed (11,16).
Advantages of RATS
The most obvious advantage of RATS over conventional cervical thyroidectomy is that it eliminates the need for any cervical incision. This cosmetic aspect makes RATS appealing especially to young female patients and those with a tendency toward keloid formation.
The RATS has several technical advantages over the open and endoscopic approaches. First, the robotic system provides three-dimensional magnified visualization, which enables an easier identification of the RLN and parathyroid glands compared to the cervical approach; Second, it eliminates the natural surgeon tremor; and, third, it enables a wider range of motion through the robot’s EndoWrist and the articulations of the arms. All of these result in minimal complication rates and excellent cancer control and functional results. In addition, the improved visualization and surgical ergonomics provide for reduced musculoskeletal discomfort to the surgeon compared with open or endoscopic surgery.
RATS was found to yield better patient outcomes, including reduced pain and increased cosmetic satisfaction, as well as lower rates of paresthesia, postoperative voice change, and swallowing discomfort (6,17).
Disadvantages of RATS
On the other hand, due to the new approach to the surrounding anatomy and the loss of tactile sensation, RATS introduces potential new complications such as tracheal and esophageal injury. Very few studies accounted for such complications and then only in a minor way with no need to convert to open thyroidectomy (OT) (1). In addition, due to the ipsilateral arm position, there is a risk of brachial plexus neuropathy. This risk can be reduced by placing the arm in a flexed overhead 90 degrees position, thereby reducing the chance of stretching the nerves. Intra-operative monitoring of the ulnar, radial, and median nerves may further reduce the possibility of brachial plexus injury, by identification of any impending damage to these nerves and enabling the patient to be repositioned (1).
Another disadvantage of RATS is the longer operative time due to the creation of the working space and the robot docking. However, several studies have examined the learning curves of the RT and have shown that increased experience led to decreased total operative time (1). RATS involves a steep learning curve, compared to the conventional approach. However, it has been demonstrated that compared to the endoscopic approach which requires 55-60 procedures, the RT required only 35-40 procedures (6). Park et al. examined the learning curves of surgeons with little or no experience, performing transaxillary RT on 125 patients. They showed excellent results compared to those in a larger series of more experienced surgeons and specifically, that the operation times gradually decreased, reaching a plateau after 20 procedures (18). Another disadvantage of RATS is the limitation in the body habitus and BMI. While obese patients (BMI >30) make the operation (particularly the working space preparation) challenging, it has been demonstrated that, in skilled hands, this obstacle can safely be overcome (1,19,20).
In terms of cost, the RT is a more expensive procedure compared to the OT, due to the cost of the equipment and the longer operative time. However, some studies have pointed out that RT eliminated the need for an additional surgical assistant, and, combined with the potentially shorter hospital stay and the expected decrease in the maintenance cost of the robot, this may eventually result in an equally cost-effective procedure.
RATS in papillary thyroid carcinoma
The incidence of thyroid cancer is increasing worldwide, and so does the proportion of papillary thyroid microcarcinoma. Since early-stage PTC has an excellent prognosis, the patients quality of life aspect, including cosmetic concerns, may be emphasized (8,21). In 2011 Lee et al. published their experience with RT on 1,043 patients with low-risk well-differentiated thyroid carcinoma. They showed that the RATS was feasible and offered outcomes similar to conventional and endoscopic thyroidectomies. This study included several surgeons, including junior ones, from a number of medical centers (22). Another study published recently, explored the efficacy of RATS in North American population with thyroid cancer, compared to the conventional approach—they found similar operative times and blood loss, with negative margins for malignancy and similar thyroglobulin levels (3).
Ban et al. have described the surgical complications in their experience of 3,000 patients who underwent RT for thyroid cancer. Hypocalcemia was the most common complication—1% permanent; RLN injury—0.27% permanent; tracheal injury—0.2%; carotid artery injury—0.03%, skin flap injury—0.1% and brachial plexopathy in 0.13%. The mortality rate was 0% (23). Male gender, overweight BMI, a large thyroid gland and coexistent thyroiditis, are factors that were found to adversely affect the surgical outcome of RT in DTC cases, namely longer operative times (8).
The resection of the contralateral thyroid lobe in total or subtotal thyroidectomy is challenging via a single axillary incision. Therefore some surgeons doubted the surgical completeness of the RATS. A recently published meta-analysis, compared the surgical completeness and oncological outcome between RT and conventional OT in low-risk DTC. Ten studies were analyzed, including 752 patients who had RT and 1,453 patients who had OT. RT was associated with fewer central lymph nodes retrieval and less-complete resection (based on Tg levels), compared to OT, probably due to residual tissue in the contralateral side. Nevertheless, no locoregional recurrence was found in the RT group, therefore, the authors concluded that using RT was unlikely to compromise the outcomes of low-risk DTC (9). Several other studies investigated the completeness of the thyroidectomy, comparing it to conventional thyroidectomy using stimulated thyroglobulin levels, RAI uptake, and postoperative sonography. These studies ultimately demonstrated that the surgical completeness of RT is comparable to conventional thyroidectomy, if performed by experienced surgeons (21,24-27).
RATS experience
A meta-analysis comparing surgically related complications between robotic-assisted thyroidectomy (both BABA and RATS) and conventional OT summarized 11 studies, including 2,375 patients (1,536 of whom underwent RT), and concluded that RT had a longer operating time, longer hospital stay, and higher risk of temporary RLN injury than OT, but had comparable permanent complications and overall morbidity (28). Another meta-analysis published in 2014 by Jackson et al. (1) summarized a total of nine studies with 2,881 patients, 1,122 of whom underwent RT. They conclude that RT is as effective as endoscopic and OT, with equivalent post-operative results, shorter hospitalization, and higher patient satisfaction. Lee et al. have also published their experience with 2,014 patients who underwent RATS, with a low complication rate of 1% for major complications (e.g., permanent RLN or brachial injury, conversion) and 19% for minor ones (transient hypocalcemia, seroma, etc.). Interestingly, this group also compared the surgeons’ perspectives on the musculoskeletal ergonomic parameters associated with RATS and endoscopic and open surgery. They concluded that RATS resulted in less neck and back discomfort than did the other approaches (28).
RATS is being practiced mainly in South Korea and Europe and, to a smaller extent, in the US. The authors experience in The American Hospital in Paris, France, is very promising, with 212 RATS from 2010 to 2013. The procedures included 110 total thyroidectomies, 90 partial thyroidectomies, 12 parathyroidectomies, and 17 central node dissections. The average age was 45 years (range, 20-84 years), the ratio of male to female was 1:7 and the average BMI was 23 (range, 15-40). The total operative time for partial thyroidectomy was 140 minutes, and 170 minutes for a total thyroidectomy. They reported only 4 (2%) conversions to open surgery, 4 revision surgeries (2%), 1% permanent RLN injury, no permanent brachial plexus injury (4% were transient and resolved in 4-8 weeks), and no cases of permanent hypocalcemia. It should be noted that 57% of patients had large-volume thyroid glands (whose volumes according to preoperative sonography or final pathology were over 20 mL). RLN monitoring was implemented in all patients. Hospital stay did not differ from conventional thyroidectomy patients, and neither did the amount of blood loss. There were no cases of esophageal or tracheal injuries. With careful patient selection and a detailed explanation of the possible complications, we found high rates of patient satisfaction.
One of the relative contra-indications of the robotic-assisted thyroidectomy is Grave’s disease patients, due to the usually large-volume glands and hypervascularity. However, some surgeons have already reported their positive experience with Grave’s patients showing similar complication rates, blood loss and hospital stay. It should be noted that all patients received Potassium Iodide preoperatively (29,30). In skillful hands, RATS can be feasible and safe for patients with large-volume thyroid glands such as Grave’s and MNG patients.
A newly reported use of the RATS for modified radical neck dissection (MRND) suggests that the precise movements and magnified 3D vision enable a meticulous and safe dissection with recovery of similar numbers of lymph nodes as an open procedure (22,27).
Conclusions
The cervical approach is currently the “gold standard” procedure for thyroidectomy. However, in skilled hands, RATS is considered a safe alternative and should be presented to patients, especially those with aesthetic concerns. Terris stated that “We are in a period where one size no longer fits all” (4)—there is a diversity of different approaches, and the surgeon should tailor the procedure to the patient’s disease, general state, and desires. It is the surgeon’s obligation to introduce the patient to the different surgical options and consult him on the most appropriate one. With increasing experience and continued improvement in the robotic technology, the indications for RT will continue to evolve (6). The use of the robot for neck dissection via a transaxillary incision will continue to evolve and the indications to perform RATS will continue to expand. RATS should be performed in high-volume centers, by skilled surgeons. As with any new emerging technique, careful patient selection is crucial, and further evidence must be sought to confirm its indications over time.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jackson NR, Yao L, Tufano RP, et al. Safety of robotic thyroidectomy approaches: meta-analysis and systematic review. Head Neck 2014;36:137-43. [PubMed]
- Miccoli P, Bellantone R, Mourad M, et al. Minimally invasive video-assisted thyroidectomy: multiinstitutional experience. World J Surg 2002;26:972-5. [PubMed]
- Noureldine SI, Jackson NR, Tufano RP, et al. A comparative North American experience of robotic thyroidectomy in a thyroid cancer population. Langenbecks Arch Surg 2013;398:1069-74. [PubMed]
- Terris DJ. Surgical approaches to the thyroid gland: which is the best for you and your patient? JAMA Otolaryngol Head Neck Surg 2013;139:515-7. [PubMed]
- Ikeda Y, Takami H, Sasaki Y, et al. Clinical benefits in endoscopic thyroidectomy by the axillary approach. J Am Coll Surg 2003;196:189-95. [PubMed]
- Lee J, Kang SW, Jung JJ, et al. Multicenter study of robotic thyroidectomy: short-term postoperative outcomes and surgeon ergonomic considerations. Ann Surg Oncol 2011;18:2538-47. [PubMed]
- Kuppersmith RB, Holsinger FC. Robotic thyroid surgery: an initial experience with North American patients. Laryngoscope 2011;121:521-6. [PubMed]
- Son H, Park S, Lee CR, et al. Factors contributing to surgical outcomes of transaxillary robotic thyroidectomy for papillary thyroid carcinoma. Surg Endosc 2014;28:3134-42. [PubMed]
- Lang BH, Wong CK, Tsang JS, et al. A systematic review and meta-analysis evaluating completeness and outcomes of robotic thyroidectomy. Laryngoscope 2015;125:509-18. [PubMed]
- Chung WY. Pros of robotic transaxillary thyroid surgery: its impact on cancer control and surgical quality. Thyroid 2012;22:986-7. [PubMed]
- Lin HS, Folbe AJ, Carron MA, et al. Single-incision transaxillary robotic thyroidectomy: challenges and limitations in a North American population. Otolaryngol Head Neck Surg 2012;147:1041-6. [PubMed]
- Perrier ND, Randolph GW, Inabnet WB, et al. Robotic thyroidectomy: a framework for new technology assessment and safe implementation. Thyroid 2010;20:1327-32. [PubMed]
- Lee YM, Yi O, Sung TY, et al. Surgical outcomes of robotic thyroid surgery using a double incision gasless transaxillary approach: analysis of 400 cases treated by the same surgeon. Head Neck 2014;36:1413-9. [PubMed]
- Landry CS, Grubbs EG, Morris GS, et al. Robot assisted transaxillary surgery (RATS) for the removal of thyroid and parathyroid glands. Surgery 2011;149:549-55. [PubMed]
- Nam KH, Owen R, Inabnet WB 3rd. Prevention of complications in transaxillary single-incision robotic thyroidectomy. Thyroid 2012;22:1266-74. [PubMed]
- Holsinger FC, Chung WY. Robotic thyroidectomy. Otolaryngol Clin North Am 2014;47:373-8. [PubMed]
- Sun GH, Peress L, Pynnonen MA. Systematic review and meta-analysis of robotic vs conventional thyroidectomy approaches for thyroid disease. Otolaryngol Head Neck Surg 2014;150:520-32. [PubMed]
- Park JH, Lee J, Hakim NA, et al. Robotic thyroidectomy learning curve for beginning surgeons with little or no experience of endoscopic surgery. Head Neck 2014. [Epub ahead of print]. [PubMed]
- Kandil E, Abdelghani S, Noureldine SI, et al. Transaxillary gasless robotic thyroidectomy: a single surgeon's experience in North America. Arch Otolaryngol Head Neck Surg 2012;138:113-7. [PubMed]
- Kandil EH, Noureldine SI, Yao L, et al. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg 2012;214:558-64. [PubMed]
- Lee S, Lee CR, Lee SC, et al. Surgical completeness of robotic thyroidectomy: a prospective comparison with conventional open thyroidectomy in papillary thyroid carcinoma patients. Surg Endosc 2014;28:1068-75. [PubMed]
- Lee J, Yun JH, Nam KH, et al. Perioperative clinical outcomes after robotic thyroidectomy for thyroid carcinoma: a multicenter study. Surg Endosc 2011;25:906-12. [PubMed]
- Ban EJ, Yoo JY, Kim WW, et al. Surgical complications after robotic thyroidectomy for thyroid carcinoma: a single center experience with 3,000 patients. Surg Endosc 2014;28:2555-63. [PubMed]
- Tae K, Song CM, Ji YB, et al. Comparison of surgical completeness between robotic total thyroidectomy versus open thyroidectomy. Laryngoscope 2014;124:1042-7. [PubMed]
- Yi O, Yoon JH, Lee YM, et al. Technical and oncologic safety of robotic thyroid surgery. Ann Surg Oncol 2013;20:1927-33. [PubMed]
- Lee S, Ryu HR, Park JH, et al. Excellence in robotic thyroid surgery: a comparative study of robot-assisted versus conventional endoscopic thyroidectomy in papillary thyroid microcarcinoma patients. Ann Surg 2011;253:1060-6. [PubMed]
- Lee J, Kwon IS, Bae EH, et al. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J Clin Endocrinol Metab 2013;98:2701-8. [PubMed]
- Lang BH, Wong CK, Tsang JS, et al. A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol 2014;21:850-61. [PubMed]
- Kwon H. Bilateral axillo-breast approach robotic thyroidectomy for Graves' disease: an initial experience in a single institute. World J Surg 2013;37:1576-81. [PubMed]
- Noureldine SI, Yao L, Wavekar RR, et al. Thyroidectomy for Graves’ disease: a feasibility study of the robotic transaxillary approach. ORL J Otorhinolaryngol Relat Spec 2013;75:350-6. [PubMed]


