Clinicopathological review of pancreatoblastoma in adults
Introduction
Pancreatoblastoma is a rare malignant epithelial neoplasm of the pancreas with demonstrated multiple lines of differentiation. Infantile pancreatic carcinoma was the term previously used to describe this neoplasm. Horie et al. (1) proposed the term pancreatoblastoma because of the histological semblance of the tumor to fetal pancreatic tissue consistent with gestational age of 7 weeks. The term pancreatoblastoma has since been widely accepted.
A retrospective review of patients under 21 years of age managed at the Memorial Sloan-Kettering Cancer Centre for malignant pancreatic tumors spanning a period of 35 years identified a total of 5 pancreatoblastomas out of 17 malignant pancreatic neoplasms, thus underscoring the rarity of this tumor (2). PB is commonly seen in the pediatric population where it accounts for approximately 25% of childhood pancreatic neoplasms (2,3). Approximately 200 cases of PB reported in the literature since 1957 when it was first described by Becker, have been mostly reported in children with very few cases in the adult population (4).
This paper reviews the very rare cases of adult pancreatoblastoma reported in the literature with a discussion of the clinicopathological features, treatment modalities and management outcomes.
Methods
The case report and case series of adult (aged 18 and above) pancreatoblastoma were retrieved from PubMed, Scopus and relevant databases. All studies retrieved were assessed for epidemiologic, clinical, histopathological, treatment and follow up data. The search terms were adult pancreatoblastoma, pancreatic neoplasms, infantile pancreatic carcinoma etc.
Results
Tables 1,2 present a summary of the clinicopathological features and treatment outcome of the 35 cases of adult pancreatoblastoma in the literature.
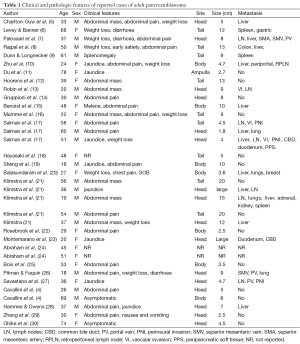
Full table
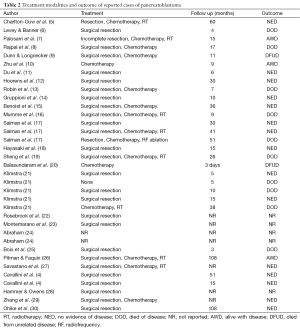
Full table
Discussion
Adult PB is a very rare primary tumor of the pancreas. Twenty nine years after the first case of adult pancreatoblastoma was reported by Palosaari et al. (7), to the best of the knowledge of the author, 35 cases have so far been reported in the literature.
The mean age of presentation was 41 years (range, 18-78 years). The male sex accounted for 51.4% of the cases in the literature which suggests an almost equal affectation between the male and female sex. This finding is inconsistent with earlier observation of male predominance of pancreatoblastoma in adults (4,22). The tumor seems to have a predilection for the head of the pancreas which accounted for approximately 49% of the cases reviewed. This finding is in contrast to earlier reports that suggested no preferential tumor site of origin in the pancreas (11,22,28). The tail and the body of the pancreas accounted for 27% and 21% of tumor locations respectively. There was one case of ampullary pancreatoblastoma in a 78-year-old lady (11).
Pancreatoblastoma is an indolent neoplasm with a non-specific clinical presentation. Abdominal pain (4,5,7,10,14-17,19,21,22,25,26,28,29) was the commonest clinical symptom and it accounted for 50% of the presentation in adult PB. Weight loss (5-8,10,17,20,21,26) and abdominal mass (5,12,13,16,21) accounted for 28% and 22% of the presentation respectively. Other symptoms include jaundice (10,11,19,21,23,27,28), diarrhea (6,7,26), gastrointestinal bleeding (15) and splenomegaly (9). Two cases were asymptomatic (4,30). Clinical data was not available for three cases (18,24).
The etiology and the molecular pathogenesis of PB is unknown. The tumor mostly occurs sporadically, however association with genetic syndromes such as familial adenomatous polyposis syndrome (24) and Beckwith-Weidemann syndrome (31,32) have been documented. The possibility of a potential molecular similarity and genetic alterations between PB and hepatoblastoma based on the observation of the association of both tumors with Beckwith-Weidemann syndrome was investigated by Abraham et al. (24). The findings suggested significant and frequent (86%) allelic loss on 11p and somatic mutations in the APC/B-catenin pathway in the pancreatoblastoma examined which is consistent with genetic alterations found in hepatoblastoma. In contrast to most pancreatic ductal adenocarcinomas, pancreatoblastoma does not seem to exhibit p53 and k-ras genetic alterations (24).
Elevated tumor markers such as CEA and AFP levels have been documented in 30-50% of PB in the pediatric population (4), however, elevation of serum tumor markers are not consistent and generally not helpful in the diagnosis of adult PB. Elevated CA 19-9 which is used as a tumor marker for ductal adenocarcinoma of the pancreas have been reported in two cases of adult PB (11,20). CA19-9 is also known to be elevated in hepatocellular carcinoma, colorectal ca, obstruction of the biliary system etc. A case of concurrent AFP producing, enzyme producing (serum lipase) and hormone producing (proinsulin) in adult PB (8) have been documented. Corticotrophin releasing hormone in adult PB was also reported by Boix et al. (25).
Pre-operative diagnosis with FNA is difficult because of the multiple lines of differentiation exhibited by PB which also overlaps with other tumors such as acinar cell carcinoma of the pancreas. So far two cases of adult PB have been diagnosed with FNA cytology in combination with other ancillary studies (10,26). The diagnostic challenge also arises because the distinguishing histological feature of PB—squamoid corpuscles may not be sampled by FNA simply due to sampling error.
There is no difference in the imaging findings of PB in both adult and pediatric populations (22). Montemarano et al. in a large review of imaging findings in patients with PB suggested that PB are large, well defined and fairly well circumscribed enhanced heterogeneous masses with low to intermediate signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Rim-shaped or clustered calcifications may be present (23). Lee et al. in a review of US and CT findings in PB suggested a solid inseparable pancreatic mass with mixed echogenicity as the most common finding on US and a well-defined large multiloculated mass with enhancing septa on CT as a typical finding in PB (33). Atypical findings such as a small sized tumor (2.5 cm) with a well-defined rim of soft tissue and demonstrated Doppler flow and contrast enhancement on CT have been reported. The rim on dynamic gadolinium-enhanced MRI imaging demonstrated rapid arterial phase enhancement with late washout which is suggestive of its vascular nature. In contrast the mass showed central areas of high T2 signal intensity without enhancement (22).
PB is known for the demonstrable multiple lines of differentiation which includes more prominently acinar with foci of ductal, squamous, endocrine and rarely mesenchymal differentiation (8,21). Grossly PB is often well-defined and partially encapsulated large tumors with an average size of 8 cm (range, 1.8-20 cm). PB are mostly solid, lobulated, fleshy and solitary tumors which are tan, gray or whitish- yellow in color with a soft consistency on cut sections (11,27-29). Rarely the tumor may have predominantly cystic component which has been observed in tumors associated with Beckwith-Weidemann syndrome (22,31). PB have well-defined lobules separated by dense hyper cellular fibrous band or stroma with foci of necrosis (29) or extensive central tissue degeneration and necrosis (22).
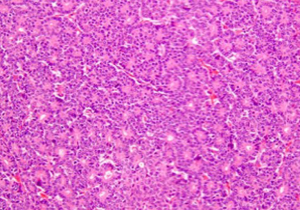
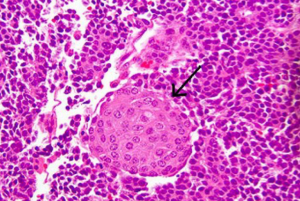
PB demonstrates an appearance of geographic nests of light and dark staining cells indicative of the multiple cellular differentiations observed in the tumor. Some of the dark staining cells had appearance suggestive of acinar differentiation with small nuclei which has prominent nucleoli and amphophilic to granular eosinophilic cytoplasm (3,28) (Figure 1). In some areas, some cells may demonstrate an appearance suggestive of neuroendocrine differentiation with basophilic nuclei and scant cytoplasm (28). Squamoid corpuscles (Figure 2) which is the most characteristic and distinguishing feature of PB are demonstrated by multiple foci of light staining spindle shaped cells with a whorled nested pattern and squamous appearance or scattered islands of plump epithelioid cells (3,11). The frequency of the squamoid corpuscles varies in different areas of the tumor. Scattered mitotic figures are noted in the tumor as well (11,22,28).
The immunohistochemical staining is a reflection of the different cellular differentiation of the tumor. Markers of ductal differentiation such as CEA, B72.3 and DUPAN-2 are present in 50-65% of cases (21). The neuroendocrine component stains positive for neuroendocrine markers chromogranin, synaptophysin and neuron-specific enolase which is found in over two-thirds of cases. PB demonstrates prominent staining for markers of acinar differentiation such as trypsin, chymotrypsin and less commonly lipase (4,21,29). Ultrastructural studies of PB have confirmed the heterogeneity of cellular differentiation in the tumor by showing zymogen and neuroendocrine granules (4).
PB invades adjacent structures such as the spleen, colon, duodenum, portal vein, superior mesenteric vessels, peripancreatic soft tissue, common bile duct and perineural infiltration. Metastasis and or invasion of adjacent structures accounted for 58% of adult PB. The liver is the commonest site for metastasis. Other sites include lymph nodes, lungs, bone and peritoneum. A very rare case of metastatic spread to the breast has been documented (20).
Surgical resection is the mainstay of treatment and complete resection has been associated with long term survival (21). A case with metastasis to the liver who had a combination of surgical resections (distal pancreatectomy, splenectomy and wedge resections of the liver metastatic deposits) and 6 cycles of adjuvant chemotherapy with no evidence of disease at 36 months of follow up, highlights the need for aggressive surgical management of the tumor (15). Chemotherapy with or without surgical resection have been used in the treatment of adult PB (5,7-10,13,15-17,19-21,26,27,29) with variable outcomes. Cases of combination therapy with radiotherapy have been documented (5,7,16,17,19,21,26,27) as well. The median follow up for patients with readily available data was 15 months (range, 1-108 months) and the longest documented survival was 108 months in two cases (26,30). About 40% of the patients were dead at follow-up, 50% of the cases had no evidence of disease and three patients were alive with disease at follow-up. Adult patients with PB generally have poorer prognosis compared to pediatric patients (4,8). The prognosis seems to be good for patients with resectable tumors without metastasis. It is however poor for patients with unresectable tumor and widespread metastasis (21,23).
In conclusion, pancreatoblastoma is a malignant tumor of the pancreas which occurs very rarely in adults. Serum tumor markers are generally not helpful in adult PB in contrast to pediatric patients. Metastatic disease and/or local infiltration of surrounding tissues is common. PB demonstrates different cellular differentiation and hence presents a diagnostic challenge for preoperative diagnosis using fine needle aspiration cytology. Histopathological examination of resected specimens is necessary for accurate diagnosis. PB should be considered a differential diagnosis of solid and cystic pancreatic neoplasms. The role of adjuvant chemotherapy and radiotherapy in the management of adult PB is unclear and may be helpful especially in the management of metastatic and unresectable disease. Surgical resection of the tumor is the mainstay of therapy with a variable combination of radiotherapy and chemotherapy.
Acknowledgements
Many thanks to the Dr. Allendorf JD and the editorial office of Journal of the Pancreas for the kind permission to use the figures from the article entitled “Revisiting metastatic adult pancreatoblastoma. A case and review of the literature”.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Horie A, Yano Y, Kotoo Y, et al. Morphogenesis of pancreatoblastoma, infantile carcinoma of the pancreas: report of two cases. Cancer 1977;39:247-54. [PubMed]
- Shorter NA, Glick RD, Klimstra DS, et al. Malignant pancreatic tumors in childhood and adolescence: The Memorial Sloan-Kettering experience, 1967 to present. J Pediatr Surg 2002;37:887-92. [PubMed]
- Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol 2007;20 Suppl 1:S94-112. [PubMed]
- Cavallini A, Falconi M, Bortesi L, et al. Pancreatoblastoma in adults: a review of the literature. Pancreatology 2009;9:73-80. [PubMed]
- Charlton-Ouw KM, Kaiser CL, Tong GX, et al. Revisiting metastatic adult pancreatoblastoma. A case and review of the literature. JOP 2008;9:733-8. [PubMed]
- Levey JM, Banner BF. Adult pancreatoblastoma: a case report and review of the literature. Am J Gastroenterol 1996;91:1841-4. [PubMed]
- Palosaari D, Clayton F, Seaman J. Pancreatoblastoma in an adult. Arch Pathol Lab Med 1986;110:650-2. [PubMed]
- Rajpal S, Warren RS, Alexander M, et al. Pancreatoblastoma in an adult: case report and review of the literature. J Gastrointest Surg 2006;10:829-36. [PubMed]
- Dunn JL, Longnecker DS. Pancreatoblastoma in an older adult. Arch Pathol Lab Med 1995;119:547-51. [PubMed]
- Zhu LC, Sidhu GS, Cassai ND, et al. Fine-needle aspiration cytology of pancreatoblastoma in a young woman: report of a case and review of the literature. Diagn Cytopathol 2005;33:258-62. [PubMed]
- Du E, Katz M, Weidner N, et al. Ampullary pancreatoblastoma in an elderly patient: a case report and review of the literature. Arch Pathol Lab Med 2003;127:1501-5. [PubMed]
- Hoorens A, Gebhard F, Kraft K, et al. Pancreatoblastoma in an adult: its separation from acinar cell carcinoma. Virchows Arch 1994;424:485-90. [PubMed]
- Robin E, Terris B, Valverde A, et al. Pancreatoblastoma in adults. Gastroenterol Clin Biol 1997;21:880-3. [PubMed]
- Gruppioni F, Casadei R, Fusco F, et al. Adult pancreatoblastoma. A case report. Radiol Med 2002;103:119-22. [PubMed]
- Benoist S, Penna C, Julié C, et al. Prolonged survival after resection of pancreatoblastoma and synchronous liver metastases in an adult. Hepatogastroenterology 2001;48:1340-2. [PubMed]
- Mumme T, Büttner R, Peiper C, et al. Pancreatoblastoma: a rare malignant neoplasm in early adulthood. Chirurg 2001;72:806-11. [PubMed]
- Salman B, Brat G, Yoon YS, et al. The diagnosis and surgical treatment of pancreatoblastoma in adults: a case series and review of the literature. J Gastrointest Surg 2013;17:2153-61. [PubMed]
- Hayasaki N, Miyake N, Takahashi H, et al. A case of pancreatoblastoma in an adult. Nihon Shokakibyo Gakkai Zasshi 1999;96:558-63. [PubMed]
- Sheng L, Weixia Z, Longhai Y, et al. Clinical and biologic analysis of pancreatoblastoma. Pancreas 2005;30:87-90. [PubMed]
- Balasundaram C, Luthra M, Chavalitdhamrong D, et al. Pancreatoblastoma: a rare tumor still evolving in clinical presentation and histology. JOP 2012;13:301-3. [PubMed]
- Klimstra DS, Wenig BM, Adair CF, et al. Pancreatoblastoma. A clinicopathologic study and review of the literature. Am J Surg Pathol 1995;19:1371-89. [PubMed]
- Rosebrook JL, Glickman JN, Mortele KJ. Pancreatoblastoma in an adult woman: sonography, CT, and dynamic gadolinium-enhanced MRI features. AJR Am J Roentgenol 2005;184:S78-81. [PubMed]
- Montemarano H, Lonergan GJ, Bulas DI, et al. Pancreatoblastoma: imaging findings in 10 patients and review of the literature. Radiology 2000;214:476-82. [PubMed]
- Abraham SC, Wu TT, Klimstra DS, et al. Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am J Pathol 2001;159:1619-27. [PubMed]
- Boix E, Yuste A, Meana A, et al. Corticotropin-releasing hormone-secreting pancreatoblastoma in an adult patient. Pancreas 2010;39:938-9. [PubMed]
- Pitman MB, Faquin WC. The fine-needle aspiration biopsy cytology of pancreatoblastoma. Diagn Cytopathol 2004;31:402-6. [PubMed]
- Savastano S, d’Amore ES, Zuccarotto D, et al. Pancreatoblastoma in an adult patient. A case report. JOP 2009;10:192-5. [PubMed]
- Hammer ST, Owens SR. Pancreatoblastoma: a rare, adult pancreatic tumor with many faces. Arch Pathol Lab Med 2013;137:1224-6. [PubMed]
- Zhang D, Tang N, Liu Y, et al. Pancreatoblastoma in an adult. Indian J Pathol Microbiol 2015;58:93-5. [PubMed]
- Ohike N, Yamochi T, Shiokawa A, et al. A peculiar variant of pancreatoblastoma in an adult. Pancreas 2008;36:320-2. [PubMed]
- Kohda E, Iseki M, Ikawa H, et al. Pancreatoblastoma. Three original cases and review of the literature. Acta Radiol 2000;41:334-7. [PubMed]
- Koh TH, Cooper JE, Newman CL, et al. Pancreatoblastoma in a neonate with Wiedemann-Beckwith syndrome. Eur J Pediatr 1986;145:435-8. [PubMed]
- Lee JY, Kim IO, Kim WS, et al. CT and US findings of pancreatoblastoma. J Comput Assist Tomogr 1996;20:370-4. [PubMed]


