Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction
Introduction
Given the high prevalence and incidence of breast cancer in society (1,2) and a growing number of women with breast cancer opting for mastectomy over breast-conserving operations (3), breast reconstruction has become an important part of breast cancer management. It can improve patients’ psychosexual well-being and their overall psyche in response to breast cancer management (4-8). Autologous breast reconstruction (and in particular those with perforator-based free flaps) has demonstrated a natural-appearing, aesthetically-pleasing, long-lasting restorative option, with low donor site morbidity (9,10). Recent advancements in operative techniques and imaging modalities have facilitated complex microvascular breast reconstructions to become safer, more reliable procedures (11-13).
Various autologous tissues have been utilized for breast reconstruction, such as omentum (14), latissimus dorsi (15-18), deep circumflex iliac artery (groin) flap (19,20), lateral thigh (tensor fascia latae) flap (21), gluteal musculocutaneous flap (22-25), gracilis flap (26), and triceps flap (27). In recent times, the anterior abdominal wall has become the most frequently used donor site due to the added aesthetic benefit at the donor site, akin to a concomitant abdominoplasty. Initially, transverse rectus abdominis muscle (TRAM) flaps were successful in providing adequate volume replacement for breast reconstructions (28,29). However, a high rate of donor site morbidity, such as rectus abdominis muscle weakness and ventral hernia, resulted in the development of muscle-sparing techniques, mainly the deep inferior epigastric artery perforator (DIEP) flaps (10,30). DIEP flaps are fasciocutaneous flaps based on musculocutaneous perforators derived from the deep inferior epigastric artery (DIEA) (31,32). They were able to provide sufficient tissue volume and a superior functional and aesthetic outcome at the donor site than the TRAM flaps (12,33). However, early studies reported a steep learning curve of the microsurgical technique leading to a longer dissection time, and an increased flap complications, such as fat necrosis and flap loss (34). To this effect, the use of preoperative imaging has been instrumental.
Preoperative assessment of the donor site microvasculature anatomy with advanced imaging modalities has assisted surgeons in the appropriate selection of the donor site, perforator, and flap leading to an overall improvement in the flap outcomes (35,36). According to the consensus reached at the Navarra meeting, a perforator should be selected on the basis of its caliber, central location within the flap, direct venous connection with the main superficial venous system, and it preferably demonstrates a broach subcutaneous branching pattern and has a shorter intramuscular (IM) course for ease of dissection (37). Hence, an ideal preoperative imaging technique should accurately demonstrate the individual variations in the location and caliber of the perforators, their IM course, and the branching pattern of the DIEA (38). Early investigators have relied on handheld Doppler probes and color duplex ultrasonography to detect perforators, characterize them in flow velocity and resistivity, and create a perforator map on the abdominal wall (39-41). Both ultrasound techniques are inexpensive, do not expose patients to radiation or potentially nephrotoxic intravenous contrast agents, can detect perforators with diameter greater than 0.5 mm, identify any underlying vessel damage secondary to arthrosclerosis or previous surgery (42-45). However, they are subject to significant inter-observer variability, and are associated with poor consistency with intraoperative findings, high false positive and negative rates (39,41,46,47). Hence, they are now superseded by modern imaging technologies with objective findings, such as fluorescent angiography, computed tomographic angiography (CTA), and magnetic resonance angiography (MRA).
In this review, we evaluate the accuracy of fluorescent angiography, CTA, and MRA, and compare their impact on the clinical outcomes of patients undergoing autologous breast reconstruction, mainly TRAM and DIEP flaps, since they have attracted the most number of clinical studies and have provided the highest level of evidence (48).
Methods
We reviewed the published English literature from 1950 to 2015 from well-known databases, such as PubMed, Medline, Web of Science, and EMBASE, using search terms, such as “autologous breast reconstruction”, “DIEP flap”, “fluorescent angiography”, “computed tomographic angiography”, and “magnetic resonance angiography”.
Results
Fluorescent angiography (FA)
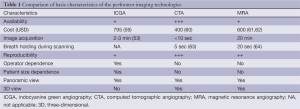
FA utilizes intravenous dyes that fluoresce and emit infrared energy upon excitation by a light source, which produces real-time videos that facilitate evaluation of the anastomotic patency and the extent of soft tissue perfusion (49,50). Originally, the investigators employed fluorescein dye, which accumulates extracellularly in the soft tissue, fluoresces upon excitation by the ultraviolet (UV) light, and is renally excreted (51,52). However, the long time it takes to reach the maximum intensity (15 minutes), relatively frequent adverse effects, reports of allergic reaction, and the steep learning curve associated with using a Woods lamp for interpretation have resulted in the fluorescein dye being replaced by the indocyanine green (ICG) dye. ICG is an FDA-approved, biliary excreted, water-soluble dye that enables image capture within 2-3 minutes of intravenous administration (53). ICG is excited by laser and transmits infrared energy that is recorded by devices equipped with inbuilt software algorithms that generate quantitative data, such as LifeCell SPY system (LifeCell Corp, Branchburg, New Jersey, USA), IC-View (Pulsion Medical Systems AG, Munich, Germany), and FLARE imaging system (Beth Israel Deaconess Medical Center, Boston, MA, USA) (54-56). Furthermore, ICG has a short half-life (3-4 minutes) (57), which enables multiple consecutive measurements, in contrast to fluorescein, which is retained in the tissues (58). It strongly binds to the plasma proteins leading to rapid washout from the circulation and has a superior side effect profile with a low rate of anaphylaxis (1 in 42,000) (Table 1) (65,66).
Full table
Laser-assisted ICGFA (LA-ICGFA) has demonstrated utility by characterizing vascular flow dynamics and tissue perfusion in various disciplines (67-75). In reconstructive surgery, investigators have utilized LA-ICGFA intraoperatively to assess the patency of microvascular anastomosis in free flaps (76,77) and calculate the intrinsic transit time through the anastomosis (78) that correlate with postoperative flap compromise and accurately predict early re-exploration. One of the significant limitations of LA-ICGFA is that it can only provide information a few millimeters deep from the skin (55). This is adequate for evaluating thin areas, such as the extremities, head and neck, and the trunk (79). However, since majority of autologous breast reconstructions are based on the abdomen and a thick pannus is preferred for a DIEP flap, LA-ICGFA has a minimal role in the preoperative planning (55). In breast reconstruction, LA-ICGFA may be useful intraoperatively during flap harvest to assess the flap perfusion, confirm blood flow within the microvascular anastomosis, and detect acute changes in the flap circulation, such as arterial occlusion, venous thrombosis, and pedicle torsion (80). Moreover, it can be used to evaluate the perfusion of mastectomy skin flaps and facilitate the reconstructive surgeon to debride areas that are likely to develop necrosis (59).
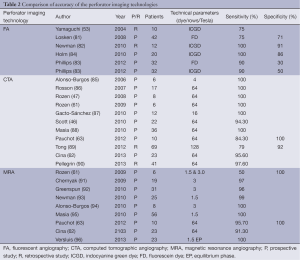
A number of studies in the literature have examined the accuracy of LA-ICGFA in estimating postoperative complications, such as mastectomy skin flap necrosis (81-83), partial flap necrosis (53) and microvascular thrombosis (Table 2) (84). Using fluorescein dye, Losken et al. reported a sensitivity and specificity of 75% and 71% respectively to detect mastectomy skin flap necrosis (81). Using ICG dye, Newman et al. retrospectively reviewed and derived that LA-ICGFA can detect postoperative skin necrosis with a sensitivity and specificity of 100% and 91% respectively (82). In a prospective study of 51 implant breast reconstructions in 32 patients, Phillips et al. compared the efficacy of fluorescein to the ICG dye and reported that both dyes have the same sensitivity of 90% in detecting skin necrosis but ICG had a slightly superior specificity (83). In a retrospective study of ten patients undergoing TRAM flaps, Yamaguchi et al. report that intraoperative LA-ICGFA can detect partial flap necrosis with a sensitivity of 75% (53). Moreover, Holm et al. have demonstrated that LA-ICGFA accurately detects microvascular thrombosis as the cause of free flap re-exploration with a sensitivity of 100% and specificity of 86% (84).
Full table
In the literature, there are only two studies where using LA-ICGFA is correlated with clinical outcomes (Table 3) (59,97). Komorowska-Timek et al. applied LA-ICGFA intraoperatively in 24 consecutive patients undergoing breast reconstruction and the areas of inadequate dye penetration suggesting poor tissue perfusion were resected (97). The authors reported that a resultant total complication rate of 4%, which was lower than 15.1% recorded from their previous 148 patients and 206 breast reconstructions (P<0.01) (97). Duggal et al. retrospectively reviewed the clinical outcomes in 184 patients undergoing breast reconstructions receiving intraoperative LA-ICGFA (59). The authors report that LA-ICGFA was associated with a significant reduction in mastectomy skin flap necrosis (P=0.01) and re-operation rate (P=0.009). There was also a trend demonstrated in the reduction of partial and complete flap loss rate (P=0.237 and P=1.00, respectively).
Full table
Computed tomographic angiography (CTA)
First reported by Masia et al. in 2006 (98), CTA is widely used for preoperative imaging and planning free tissue transfers by numerous institutions around the world and is currently considered the best of the three options due to its high accuracy and reliability (Table 1) (35,60,86,107-111). Ongoing advances in CTA, such as an increasing number of detector rows, ensure that the modality remains fast and produces high detail (48). For interpretation, the scan data can be three-dimensionally (3D) reconstructed digitally on either a free software, such as Osirix (Pixmeo, Geneva, Switzerland), or a commercially available software, such as Siemens Inspace (Siemens, Berlin, Germany). Using 3D volume rendering technique in the software facilitates the creation of a perforator location map and illustrates the subcutaneous course of the perforators (see Figure 1); and secondly, the maximum intensity projection technique can help visualize the vascular pedicle in the coronal plane (see Figure 2) and in the axial plane, which can further depict its IM course (see Figure 3) (85,113).
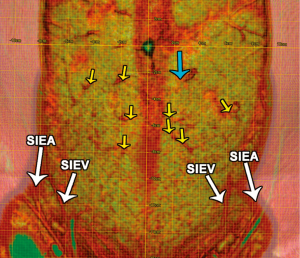
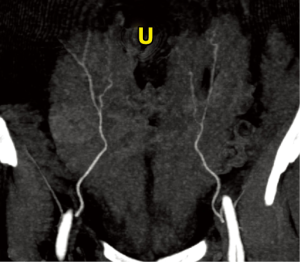
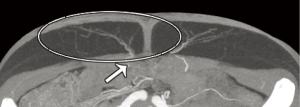
The major advantages of CTA are its wide availability, affordability, non-invasive nature, high reproducibility and operator-independence. Furthermore, it has a fast scanning time of less than 5 minutes (36) and produces images in high spatial resolution and in multiplanar or 3D panoramic views that facilitates ease of interpretation. As a result, the location, caliber, and course of musculocutaneous perforators as small as 0.3 mm in diameter can be readily displayed (47). In contrast to ultrasonography, the image quality is less affected by the body habitus (47) and it can clearly demonstrate both DIEA and superficial inferior epigastric artery (SIEA), and their branching patterns. In addition, the CTA can be used to screen for comorbidities, such as metastatic diseases, and detect any underlying abdominal wall defects (48) or other incidentally discovered lesions, such as angiomyolipoma and adrenal mass, that may alter the surgical management (89,90).
A plethora of studies have been reported in the literature demonstrating high accuracy of CTA in detecting perforators suitable for perforator-based free flap reconstructions (Table 2). Most investigators report sensitivity and specificity close to 100% (46,47,61-63,85-90). Furthermore, CTA can also characterize the DIEA branches, IM course, and both superficial and deep venous systems supporting a flap with high sensitivity (100%, 97.1%, 91.3%, 94.4%, respectively) (62). In comparison to Doppler ultrasound, Rozen et al. demonstrated that CTA produces superior visualization of the DIEA, its branching pattern, its perforators (P=0.0078), and additionally, the SIEA (47). Similarly, Scott et al. exhibit that CTA is significantly more sensitive than color Duplex ultrasound in detecting the top two perforators (94.3% vs. 66.3%, respectively) (46). Compared to the MRA, CTA has a superior fat-to-vessel contrast (P=0.007), but a poorer muscle-to-vessel contrast (P=0.001) (63). The former indicates that CTA is able to produce higher quality images of the subcutaneous course of a perforator; however, the latter signifies that MRA is technically superior at delineating the IM course of a perforator.
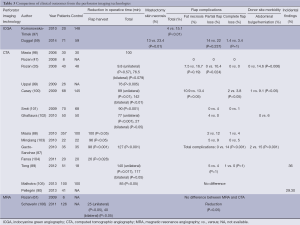
Enhanced understanding of the microvascular anatomy facilitated by CTA has assisted reconstructive surgeons in selecting an appropriate donor site, perforator, and flap, and numerous studies demonstrate that this has directly translated into an improvement in the clinical outcomes (Table 3). The studies have reported a significant reduction in the flap harvest time and the total operative time (35,87-89,98-106). This leads to reduced exposure to general anesthesia, reduced risk of infection, and reduced intraoperative bleeding (35). Furthermore, the use of CTA for preoperative planning is associated with a reduction in postoperative flap complications, such as fat necrosis, partial, and total flap loss, and donor site morbidity, such as abdominal bulge and herniation (35,47,87-89,100-103). Interestingly, one study by Malhotra et al. demonstrated no improvement in flap complications from preoperative CTA, even though there was a significant reduction in the operative time (P<0.05), intraoperative blood loss (P<0.05), and inpatient hospital stay (P<0.05) (105).
The main limitations associated with CTA stem from potential sensitivity to the iodinated intravenous contrast, contrast-induced nephrotoxicity in patients with renal impairment, and exposure to ionizing radiation. The latest CTA scanning protocols that assess a targeted area for identifying abdominal wall perforators (114) and the development of radiation dose reduction software and algorithms in the latest scanners (60,115) have decreased the average radiation exposure to 5 mSv per scan (62,98,107,111). This dose is equivalent of two abdominal X-rays, is significantly lower than a routine abdominal CT scan (63), and is theoretically associated with a 1-in-4,270 risk of fatal radiation-induced cancer (116). Moreover, perforators at the recipient site are not simultaneously imaged in order to minimize radiation. Most often, the patients have had a contrast-CT scan of the chest wall for their original breast cancer staging. Nonetheless, the recipient vessels, most commonly the internal mammary perforators, can be adequately visualized using a handheld Doppler probe (114). Furthermore, thoracic imaging poses risk to the radiation-sensitive contralateral breast and thyroid.
Magnetic resonance angiography (MRA)
Recently, MRA with Gadolinium-based contrast has become popular in order to bypass the risk of radiation associated with CTA (Table 1) (61). Recent advances in the image acquisition technique, introduction of novel contrast agents, and increasing availability of MRI scanners with stronger field strength have significantly improved the accuracy and the quality of MRA images (117). Delayed equilibrium phase (EP) technique acquires images when both the artery and the vein are enhancing, compared to the conventional first-pass, or arterial-phase, technique (96). As a result, EP facilitates a longer image acquisition time leading to higher spatial and contrast resolution, produces diagnostic quality data despite minor motion artifacts, and has 100% sensitivity in detecting abdominal perforators (96). In addition, investigators have reported prone position to minimize respiratory-related motion artifacts (92,118,119). However, this method remains controversial since it alters the natural curved anatomy of the abdomen compromising the image quality of the perforators and since patients are indeed operated in supine position (62).
In contrast to the conventional gadolinium contrast agents, extracellular contrast agents, such as gadobenate dimeglumine, offer slightly higher relaxivity (120). However, it only has a short half-life of 100 seconds (120). Newer blood pool contrast agents, mainly gadofosveset trisodium (121), has demonstrated superior quality images secondary to a longer imaging window and a relatively large R1 (122). Gadofosveset trisodium has a long half-life of 28 minutes and reversibly binds to serum albumin with high fraction (90%) (123) leading to stronger contrast enhancement of the vessels (124,125). Stronger field strength 3.0 T scanners are increasingly becoming commonplace. They demonstrate superior spatial resolution and augment gadolinium-based contrast enhancements with reduced acquisition time and a decreased susceptibility to motion artifacts (126-129).
One of the significant benefits of MRA is that it eliminates exposure to ionizing radiation. Furthermore, gadolinium-based contrast agents have a safer risk profile, such as the rate of acute allergic reaction (0.07% vs. 3%), in comparison to radioactive contrasts (130,131). Thus, MRA may be advantageous in patients with younger age, iodine allergy, and impaired renal function. Moreover, muscle-to-vessel contrast ratio is superior in MRA, compared to CTA, leading to a clearer depiction of the perforator IM course (63). In autologous breast reconstructions, there are a growing number of reports demonstrating its accuracy in delineating perforators and its potential role in improving clinical outcomes.
Despite high specificity (100%), Rozen et al. reported in an earlier study that MRA has low sensitivity (50%) in detecting abdominal wall perforators for breast reconstruction, suggesting it as an inferior option to CTA for perforator mapping purposes (see Figure 4) (61). Advances in the imaging technique, contrast agents, and the application of higher field strength scanners have improved its accuracy in the last decade (Table 3) (36). As a result, more recent studies report a high sensitivity (91.3% to 100%) with MRA (62,63,91-96). Of note, the accuracy of IM course depiction is high with MRA (62,93-95). In contrast to CTA, there is a relative paucity in the literature describing MRA for a large clinical series describing its impact on clinical outcomes. Schaverien et al. report that in 126 patients, MRA reduced the rate of partial flap loss (P<0.05) and the total operative time in both unilateral and bilateral cases by 25 and 40 minutes, respectively (106). However, the latter did not reach statistical significance. In an early study, Rozen et al. demonstrated that using MRA reduced the incidence of flap complications to 0% in six patients (61).

One of the major drawbacks of MRA is related to its relatively high cost and low availability since an average MRA scan costs USD 600, compared to USD 400 for a CTA (61). Furthermore, due to its poor spatial resolution, MRA is limited at detecting perforators smaller than 0.8 mm in diameter (61). However, the recent introduction of novel contrast agents (132) and higher field strength scanners (133) are expected to improve on this limitation. Moreover, due to an expanded examination window, MRA is more susceptible to motion artifacts and requires the patients to breath-hold for a long period of time (64). Despite its safer profile compared to ionizing contrast agents, gadolinium-based agents still presents with adverse effects, such as nephrogenic systemic fibrosis (134-137). Only 200 cases have been reported worldwide and this appears to be predisposed in patients with underlying impaired renal function. In addition, MRA is absolutely contraindicated in patients with severe obesity, implanted defibrillator or a pacemaker, implanted ferromagnetic device, and a cochlear implant. It is relatively contraindicated in patients with artificial heart valves and other types of implants. It is difficult to perform in patients with claustrophobia, severe anxiety, and confusion who are unable to lie still.
Discussion
Breast cancer is the most common cancer worldwide and is associated with the most common cancer-related deaths in women worldwide (2,138). Since an increasingly number of women opt for mastectomy (3), postmastectomy breast reconstruction has become an essential component of the holistic treatment in patients with breast cancer to ensure their psychosexual wellbeing. To this end, breast reconstruction with autologous tissue has been demonstrated to provide the most functional and aesthetically pleasing outcome. Abdominal wall-based, rectus muscle-sparing DIEP flaps are considered the gold standard since they provide ample volume without causing significant donor site morbidity (10,30). However, DIEP flaps are associated with longer microsurgical dissection leading to longer operative times and an increase in the postoperative microvascular complications.
To this effect, preoperative planning with modern imaging technology has become a crucial component of fashioning a DIEP flap for breast reconstruction. Handheld Doppler probes and color Duplex ultrasound are the first modality to be adapted for use in the preoperative setting (45). Although widely available and affordable, Doppler ultrasound is not sensitive or specific enough to be reliable and used routinely (108). Furthermore, it is susceptible to inter-observer variability and is unable to illustrate SIEA anatomy (46). Fluorescent angiography has been studied to preoperatively delineate the caliber and the location of the perforators (139). However, since this technology is only able to provide information up to a few millimeters deep from the skin and thick abdominal pannus is preferred in DIEP flaps, it has become less frequently used preoperatively (55). Instead, investigators are now using LA-ICGFA to assess microvascular anastomotic patency intraoperatively and evaluate perfusion in mastectomy skin flap (55,77).
Since CTA was first reported for breast reconstruction by Masia et al. (98), it has become the preferred preoperative imaging modality due to its high accuracy and reliability (38,88,108). With a free software, 3D images of the perforator anatomy can be created, from which its caliber, location, subcutaneous branching pattern, the DIEA and the SIEA anatomy can be easily visualized (113,140). However due to concerns surrounding radiation exposure, high-risk contrast agents, and contrast-related nephrotoxicity, MRA has been investigated recently as an alternative (61,95). Despite early findings suggesting low sensitivity in detecting perforators (61), recent advances in the image acquisition technique, the introduction of higher quality contrast agents, and availability of stronger 3.0 T scanners have enhanced the quality of perforator imaging from MRA (36,92,132). However, the image quality of CTA remains superior to the latest MRA technology. As a result, the latter has currently only preferred for a subset of patients in the younger age group, with iodine allergy and impaired renal function.
Conclusions
Preoperative imaging is an essential component of planning postmastectomy autologous breast reconstructions with DIEP flaps. Fluorescent angiography technology has been investigated as a preoperative imaging tool in the past. However, the investigators have demonstrated that it may instead be a useful intraoperative adjunct to evaluate the patency of microvascular anastomosis and the mastectomy skin perfusion. Currently, CTA is and remains the gold standard preoperative imaging modality due to its high accuracy, sensitivity, and specificity. In order to eliminate the radiation risk from CTA and the toxicity from radiosensitive contrast agents, MRA has been investigated in its role. Despite recent advancements, the image quality of MRA is still inferior to CTA and its widespread use is limited by high cost and lack of availability. Hence, MRA is best reserved for a subset of patients who are at a high risk from CTA, such as women with younger age, iodine allergy, and renal impairment.
Acknowledgments
Disclosure: The authors declare no conflict of interest.
References
- Rozen WM, Ashton MW. The venous anatomy of the abdominal wall for Deep Inferior Epigastric Artery (DIEP) flaps in breast reconstruction. Gland Surg 2012;1:92-110. [PubMed]
- DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52-62. [PubMed]
- Lucas DJ, Sabino J, Shriver CD, et al. Doing more: trends in breast cancer surgery, 2005 to 2011. Am Surg 2015;81:74-80. [PubMed]
- Ng SK, Hare RM, Kuang RJ, et al. Breast Reconstruction Post Mastectomy: Patient Satisfaction and Decision Making. Ann Plast Surg 2014. [Epub ahead of print]. [PubMed]
- Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 2000;106:1014-25; discussion 26-7. [PubMed]
- Nano MT, Gill PG, Kollias J, et al. Psychological impact and cosmetic outcome of surgical breast cancer strategies. ANZ J Surg 2005;75:940-7. [PubMed]
- Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer 2000;36:1938-43. [PubMed]
- Neto MS, de Aguiar Menezes MV, Moreira JR, et al. Sexuality after breast reconstruction post mastectomy. Aesthetic Plast Surg 2013;37:643-7. [PubMed]
- Kroll SS. Why autologous tissue? Clin Plast Surg 1998;25:135-43. [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [PubMed]
- Hamdi M, Khuthaila DK, Van Landuyt K, et al. Double-pedicle abdominal perforator free flaps for unilateral breast reconstruction: new horizons in microsurgical tissue transfer to the breast. J Plast Reconstr Aesthet Surg 2007;60:904-12; discussion 13-4. [PubMed]
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [PubMed]
- Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-5. [PubMed]
- Kiricuta I. The use of the great omentum in the surgery of breast cancer. Presse Med 1963;71:15-7. [PubMed]
- Schneider WJ, Hill HL Jr, Brown RG. Latissimus dorsi myocutaneous flap for breast reconstruction. Br J Plast Surg 1977;30:277-81. [PubMed]
- Muhlbauer W, Olbrisch R. The latissimus dorsi myocutaneous flap for breast reconstruction. Chir Plast 1977;4:27-34.
- Olivari N. The latissimus flap. Br J Plast Surg 1976;29:126-8. [PubMed]
- Saijo M. The vascular territories of the dorsal trunk: a reappraisal for potential flap donor sites. Br J Plast Surg 1978;31:200-4. [PubMed]
- Hartrampf CR Jr, Noel RT, Drazan L, et al. Ruben’s fat pad for breast reconstruction: a peri-iliac soft-tissue free flap. Plast Reconstr Surg 1994;93:402-7. [PubMed]
- Serafin D, Georgiade NG. Transfer of free flaps to provide well-vascularized, thick cover for breast reconstructions after radical mastectomy. Plast Reconstr Surg 1978;62:527-36. [PubMed]
- Elliott LF, Beegle PH, Hartrampf CR Jr. The lateral transverse thigh free flap: an alternative for autogenous-tissue breast reconstruction. Plast Reconstr Surg 1990;85:169-78; discussion 79-81. [PubMed]
- Fujino T, Harasina T, Aoyagi F. Reconstruction for aplasia of the breast and pectoral region by microvascular transfer of a free flap from the buttock. Plast Reconstr Surg 1975;56:178-81. [PubMed]
- Fujino T, Abe O, Enomoto K. Primary reconstruction of the breast by free myocutaneous gluteal flap. Int Adv Surg Oncol 1981;4:127-43. [PubMed]
- Shaw WW. Breast reconstruction by superior gluteal microvascular free flaps without silicone implants. Plast Reconstr Surg 1983;72:490-501. [PubMed]
- Paletta CE, Bostwick J 3rd, Nahai F. The inferior gluteal free flap in breast reconstruction. Plast Reconstr Surg 1989;84:875-83; discussion 84-5. [PubMed]
- Peek A, Muller M, Exner K. The free gracilis perforator flap for autologous breast reconstruction. Handchir Mikrochir Plast Chir 2002;34:245-50. [PubMed]
- Hartrampf CR Jr, Elliott LF, Feldman S. A triceps musculocutaneous flap for chest-wall defects. Plast Reconstr Surg 1990;86:502-9. [PubMed]
- Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg 1979;13:423-27. [PubMed]
- Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982;69:216-25. [PubMed]
- Blondeel PN, Boeckx WD. Refinements in free flap breast reconstruction: the free bilateral deep inferior epigastric perforator flap anastomosed to the internal mammary artery. Br J Plast Surg 1994;47:495-501. [PubMed]
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [PubMed]
- Onishi K, Maruyama Y, Iwahira Y. Cutaneous and fascial vasculature of the leg: anatomic study of fasciocutaneous vessels. J Reconstr Microsurg 1986;2:181-9. [PubMed]
- Bottero L, Lefaucheur JP, Fadhul S, et al. Electromyographic assessment of rectus abdominis muscle function after deep inferior epigastric perforator flap surgery. Plast Reconstr Surg 2004;113:156-61. [PubMed]
- Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg 2009;124:752-64. [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [PubMed]
- Mathes DW, Neligan PC. Current techniques in preoperative imaging for abdomen-based perforator flap microsurgical breast reconstruction. J Reconstr Microsurg 2010;26:3-10. [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. Planning and optimising DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconstr Aesthet Surg 2010;63:289-97. [PubMed]
- Rozen WM, Chubb D, Grinsell D, et al. Computed tomographic angiography: clinical applications. Clin Plast Surg 2011;38:229-39. [PubMed]
- Giunta RE, Geisweid A, Feller AM. The value of preoperative Doppler sonography for planning free perforator flaps. Plast Reconstr Surg 2000;105:2381-6. [PubMed]
- Tsukino A, Kurachi K, Inamiya T, et al. Preoperative color Doppler assessment in planning of anterolateral thigh flaps. Plast Reconstr Surg 2004;113:241-6. [PubMed]
- Blondeel PN, Beyens G, Verhaeghe R, et al. Doppler flowmetry in the planning of perforator flaps. Br J Plast Surg 1998;51:202-9. [PubMed]
- Rand RP, Cramer MM, Strandness DE Jr. Color-flow duplex scanning in the preoperative assessment of TRAM flap perforators: a report of 32 consecutive patients. Plast Reconstr Surg 1994;93:453-9. [PubMed]
- Cina A, Salgarello M, Barone-Adesi L, et al. Planning breast reconstruction with deep inferior epigastric artery perforating vessels: multidetector CT angiography versus color Doppler US. Radiology 2010;255:979-87. [PubMed]
- Hallock GG. Doppler sonography and color duplex imaging for planning a perforator flap. Clin Plast Surg 2003;30:347-57. [PubMed]
- Taylor GI, Doyle M, McCarten G. The Doppler probe for planning flaps: anatomical study and clinical applications. Br J Plast Surg 1990;43:1-16. [PubMed]
- Scott JR, Liu D, Said H, et al. Computed tomographic angiography in planning abdomen-based microsurgical breast reconstruction: a comparison with color duplex ultrasound. Plast Reconstr Surg 2010;125:446-53. [PubMed]
- Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16. [PubMed]
- Pratt GF, Rozen WM, Chubb D, et al. Preoperative imaging for perforator flaps in reconstructive surgery: a systematic review of the evidence for current techniques. Ann Plast Surg 2012;69:3-9. [PubMed]
- Benson RC, Kues HA. Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol 1978;23:159-63. [PubMed]
- Eren S, Rubben A, Krein R, et al. Assessment of microcirculation of an axial skin flap using indocyanine green fluorescence angiography. Plast Reconstr Surg 1995;96:1636-49. [PubMed]
- O’goshi K, Serup J. Safety of sodium fluorescein for in vivo study of skin. Skin Res Technol 2006;12:155-61. [PubMed]
- Pang CY, Neligan P, Nakatsuka T, et al. Assessment of the fluorescein dye test for prediction of skin flap viability in pigs. J Surg Res 1986;41:173-81. [PubMed]
- Yamaguchi S, De Lorenzi F, Petit JY, et al. The “perfusion map” of the unipedicled TRAM flap to reduce postoperative partial necrosis. Ann Plast Surg 2004;53:205-9. [PubMed]
- Pestana IA, Coan B, Erdmann D, et al. Early experience with fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg 2009;123:1239-44. [PubMed]
- Pestana IA, Zenn MR. Correlation between abdominal perforator vessels identified with preoperative CT angiography and intraoperative fluorescent angiography in the microsurgical breast reconstruction patient. Ann Plast Surg 2014;72:S144-9. [PubMed]
- Lee BT, Hutteman M, Gioux S, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction. Plast Reconstr Surg 2010;126:1472-81. [PubMed]
- Meijer DK, Weert B, Vermeer GA. Pharmacokinetics of biliary excretion in man. VI. Indocyanine green. Eur J Clin Pharmacol 1988;35:295-303. [PubMed]
- Kuebler WM, Sckell A, Habler O, et al. Noninvasive measurement of regional cerebral blood flow by near-infrared spectroscopy and indocyanine green. J Cereb Blood Flow Metab 1998;18:445-56. [PubMed]
- Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthet Surg J 2014;34:61-5. [PubMed]
- Rozen WM, Ashton MW, Whitaker IS, et al. The financial implications of computed tomographic angiography in DIEP flap surgery: a cost analysis. Microsurgery 2009;29:168-9. [PubMed]
- Rozen WM, Stella DL, Bowden J, et al. Advances in the pre-operative planning of deep inferior epigastric artery perforator flaps: magnetic resonance angiography. Microsurgery 2009;29:119-23. [PubMed]
- Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol 2013;23:2333-43. [PubMed]
- Pauchot J, Aubry S, Kastler A, et al. Preoperative imaging for deep inferior epigastric perforator flaps: a comparative study of computed tomographic angiography and magnetic resonance angiography. Eur J Plast Surg 2012;35:795-801.
- Aubry S, Pauchot J, Kastler A, et al. Preoperative imaging in the planning of deep inferior epigastric artery perforator flap surgery. Skeletal Radiol 2013;42:319-27. [PubMed]
- Speich R, Saesseli B, Hoffmann U, et al. Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med 1988;109:345-6. [PubMed]
- Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529-33. [PubMed]
- Balacumaraswami L, Abu-Omar Y, Choudhary B, et al. A comparison of transit-time flowmetry and intraoperative fluorescence imaging for assessing coronary artery bypass graft patency. J Thorac Cardiovasc Surg 2005;130:315-20. [PubMed]
- Desai ND, Miwa S, Kodama D, et al. Improving the quality of coronary bypass surgery with intraoperative angiography: validation of a new technique. J Am Coll Cardiol 2005;46:1521-5. [PubMed]
- Taggart DP, Choudhary B, Anastasiadis K, et al. Preliminary experience with a novel intraoperative fluorescence imaging technique to evaluate the patency of bypass grafts in total arterial revascularization. Ann Thorac Surg 2003;75:870-3. [PubMed]
- Sekijima M, Tojimbara T, Sato S, et al. An intraoperative fluorescent imaging system in organ transplantation. Transplant Proc 2004;36:2188-90. [PubMed]
- Wang HD, Singh DP. The use of indocyanine green angiography to prevent wound complications in ventral hernia repair with open components separation technique. Hernia 2013;17:397-402. [PubMed]
- Oda J, Kato Y, Chen SF, et al. Intraoperative near-infrared indocyanine green-videoangiography (ICG-VA) and graphic analysis of fluorescence intensity in cerebral aneurysm surgery. J Clin Neurosci 2011;18:1097-100. [PubMed]
- Murawa D, Hunerbein M, Spychala A, et al. Indocyanine green angiography for evaluation of gastric conduit perfusion during esophagectomy--first experience. Acta Chir Belg 2012;112:275-80. [PubMed]
- Flower RW. Injection technique for indocyanine green and sodium fluorescein dye angiography of the eye. Invest Ophthalmol 1973;12:881-95. [PubMed]
- Flower RW, Hochheimer BF. Indocyanine green dye fluorescence and infrared absorption choroidal angiography performed simultaneously with fluorescein angiography. Johns Hopkins Med J 1976;138:33-42. [PubMed]
- Holm C, Mayr M, Hofter E, et al. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg 2002;55:635-44. [PubMed]
- Holm C, Mayr M, Hofter E, et al. Assessment of the patency of microvascular anastomoses using microscope-integrated near-infrared angiography: a preliminary study. Microsurgery 2009;29:509-14. [PubMed]
- Holm C, Dornseifer U, Sturtz G, et al. The intrinsic transit time of free microvascular flaps: clinical and prognostic implications. Microsurgery 2010;30:91-6. [PubMed]
- Mothes H, Donicke T, Friedel R, et al. Indocyanine-green fluorescence video angiography used clinically to evaluate tissue perfusion in microsurgery. J Trauma 2004;57:1018-24. [PubMed]
- Liu DZ, Mathes DW, Zenn MR, et al. The application of indocyanine green fluorescence angiography in plastic surgery. J Reconstr Microsurg 2011;27:355-64. [PubMed]
- Losken A, Styblo TM, Schaefer TG, et al. The use of fluorescein dye as a predictor of mastectomy skin flap viability following autologous tissue reconstruction. Ann Plast Surg 2008;61:24-9. [PubMed]
- Newman MI, Samson MC, Tamburrino JF, et al. Intraoperative laser-assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. J Reconstr Microsurg 2010;26:487-92. [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [PubMed]
- Holm C, Dornseifer U, Sturtz G, et al. Sensitivity and specificity of ICG angiography in free flap reexploration. J Reconstr Microsurg 2010;26:311-6. [PubMed]
- Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, et al. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg 2006;59:585-93. [PubMed]
- Rosson GD, Williams CG, Fishman EK, et al. 3D CT angiography of abdominal wall vascular perforators to plan DIEAP flaps. Microsurgery 2007;27:641-6. [PubMed]
- Gacto-Sánchez P, Sicilia-Castro D, Gomez-Cia T, et al. Computed tomographic angiography with VirSSPA three-dimensional software for perforator navigation improves perioperative outcomes in DIEP flap breast reconstruction. Plast Reconstr Surg 2010;125:24-31. [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [PubMed]
- Tong WM, Dixon R, Ekis H, et al. The impact of preoperative CT angiography on breast reconstruction with abdominal perforator flaps. Ann Plast Surg 2012;68:525-30. [PubMed]
- Pellegrin A, Stocca T, Belgrano M, et al. Preoperative vascular mapping with multislice CT of deep inferior epigastric artery perforators in planning breast reconstruction after mastectomy. Radiol Med 2013;118:732-43. [PubMed]
- Chernyak V, Rozenblit AM, Greenspun DT, et al. Breast reconstruction with deep inferior epigastric artery perforator flap: 3.0-T gadolinium-enhanced MR imaging for preoperative localization of abdominal wall perforators. Radiology 2009;250:417-24. [PubMed]
- Greenspun D, Vasile J, Levine JL, et al. Anatomic imaging of abdominal perforator flaps without ionizing radiation: seeing is believing with magnetic resonance imaging angiography. J Reconstr Microsurg 2010;26:37-44. [PubMed]
- Newman TM, Vasile J, Levine JL, et al. Perforator flap magnetic resonance angiography for reconstructive breast surgery: a review of 25 deep inferior epigastric and gluteal perforator artery flap patients. J Magn Reson Imaging 2010;31:1176-84. [PubMed]
- Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, et al. Preoperative planning of DIEP and SGAP flaps: preliminary experience with magnetic resonance angiography using 3-tesla equipment and blood-pool contrast medium. J Plast Reconstr Aesthet Surg 2010;63:298-304. [PubMed]
- Masia J, Kosutic D, Cervelli D, et al. In search of the ideal method in perforator mapping: noncontrast magnetic resonance imaging. J Reconstr Microsurg 2010;26:29-35. [PubMed]
- Versluis B, Tuinder S, Boetes C, et al. Equilibrium-phase high spatial resolution contrast-enhanced MR angiography at 1.5T in preoperative imaging for perforator flap breast reconstruction. PLoS One 2013;8:e71286. [PubMed]
- Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010;125:1065-73. [PubMed]
- Masia J, Clavero JA, Larranaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [PubMed]
- Uppal RS, Casaer B, Van Landuyt K, et al. The efficacy of preoperative mapping of perforators in reducing operative times and complications in perforator flap breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:859-64. [PubMed]
- Casey WJ 3rd, Chew RT, Rebecca AM, et al. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast Reconstr Surg 2009;123:1148-55. [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [PubMed]
- Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg 2010;63:1597-601. [PubMed]
- Minqiang X, Lanhua M, Jie L, et al. The value of multidetector-row CT angiography for pre-operative planning of breast reconstruction with deep inferior epigastric arterial perforator flaps. Br J Radiol 2010;83:40-3. [PubMed]
- Fansa H, Schirmer S, Frerichs O, et al. Significance of abdominal wall CT-angiography in planning DIEA perforator flaps, TRAM flaps and SIEA flaps. Handchir Mikrochir Plast Chir 2011;43:81-7. [PubMed]
- Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization -- better for the patient, the surgeon, and the hospital. Clin Radiol 2013;68:131-8. [PubMed]
- Schaverien MV, Ludman CN, Neil-Dwyer J, et al. Contrast-enhanced magnetic resonance angiography for preoperative imaging of deep inferior epigastric artery perforator flaps: advantages and disadvantages compared with computed tomography angiography: a United Kingdom perspective. Ann Plast Surg 2011;67:671-4. [PubMed]
- Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the DIEA: a cadaveric study. Plast Reconstr Surg 2008;122:363-9. [PubMed]
- Rozen WM, Ashton MW, Grinsell D, et al. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery 2008;28:306-13. [PubMed]
- Clavero JA, Masia J, Larranaga J, et al. MDCT in the preoperative planning of abdominal perforator surgery for postmastectomy breast reconstruction. AJR Am J Roentgenol 2008;191:670-6. [PubMed]
- Masia J, Larranaga J, Clavero JA, et al. The value of the multidetector row computed tomography for the preoperative planning of deep inferior epigastric artery perforator flap: our experience in 162 cases. Ann Plast Surg 2008;60:29-36. [PubMed]
- Phillips TJ, Stella DL, Rozen WM, et al. Abdominal wall CT angiography: a detailed account of a newly established preoperative imaging technique. Radiology 2008;249:32-44. [PubMed]
- Rozen WM, Ashton MW. Modifying techniques in deep inferior epigastric artery perforator flap harvest with the use of preoperative imaging. ANZ J Surg 2009;79:598-603. [PubMed]
- Pacifico MD, See MS, Cavale N, et al. Preoperative planning for DIEP breast reconstruction: early experience of the use of computerised tomography angiography with VoNavix 3D software for perforator navigation. J Plast Reconstr Aesthet Surg 2009;62:1464-9. [PubMed]
- Niumsawatt V, Rozen WM, Ashton MW, et al. Angio-CT imaging of deep inferior epigastric artery and deep superior epigastric artery perforators. In: Saba L, Rozen WM, Alonso-Burgos A, et al. eds. Imaging for Plastic Surgery. London, UK: CRC Taylor and Francis Press, 2014.
- Rozen WM, Whitaker IS, Stella DL, et al. The radiation exposure of Computed Tomographic Angiography (CTA) in DIEP flap planning: low dose but high impact. J Plast Reconstr Aesthet Surg 2009;62:e654-5. [PubMed]
- Midgley SM, Einsiedel PF, Phillips TJ, et al. Justifying the use of abdominal wall computed tomographic angiography in deep inferior epigastric artery perforator flap planning. Ann Plast Surg 2011;67:457-9. [PubMed]
- Swanson EW, Hsu YC, Cheng HT. CTA and contrast-enhanced MRA are equally accurate for localizing deep inferior epigastric perforator flap arteries: A systematic review. J Plast Reconstr Aesthet Surg 2015;68:580-1. [PubMed]
- Zou Z, Kate Lee H, Levine JL, et al. Gadofosveset trisodium-enhanced abdominal perforator MRA. J Magn Reson Imaging 2012;35:711-6. [PubMed]
- Vasile JV, Newman TM, Prince MR, et al. Contrast-enhanced magnetic resonance angiography. Clin Plast Surg 2011;38:263-75. [PubMed]
- Niendorf HP, Felix R, Laniado M, et al. Gadolinium-DTPA: a new contrast agent for magnetic resonance imaging. Radiat Med 1985;3:7-12. [PubMed]
- Lauffer RB, Parmelee DJ, Dunham SU, et al. MS-325: albumin-targeted contrast agent for MR angiography. Radiology 1998;207:529-38. [PubMed]
- Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005;40:715-24. [PubMed]
- Ersoy H, Jacobs P, Kent CK, et al. Blood pool MR angiography of aortic stent-graft endoleak. AJR Am J Roentgenol 2004;182:1181-6. [PubMed]
- Klessen C, Hein PA, Huppertz A, et al. First-pass whole-body magnetic resonance angiography (MRA) using the blood-pool contrast medium gadofosveset trisodium: comparison to gadopentetate dimeglumine. Invest Radiol 2007;42:659-64. [PubMed]
- Hadizadeh DR, Gieseke J, Lohmaier SH, et al. Peripheral MR angiography with blood pool contrast agent: prospective intraindividual comparative study of high-spatial-resolution steady-state MR angiography versus standard-resolution first-pass MR angiography and DSA. Radiology 2008;249:701-11. [PubMed]
- Barth MM, Smith MP, Pedrosa I, et al. Body MR imaging at 3.0 T: understanding the opportunities and challenges. Radiographics 2007;27:1445-62; discussion 62-4. [PubMed]
- Huang BY, Castillo M. Neurovascular imaging at 1.5 tesla versus 3.0 tesla. Magn Reson Imaging Clin N Am 2009;17:29-46. [PubMed]
- Tomasian A, Salamon N, Lohan DG, et al. Supraaortic arteries: contrast material dose reduction at 3.0-T high-spatial-resolution MR angiography--feasibility study. Radiology 2008;249:980-90. [PubMed]
- Krautmacher C, Willinek WA, Tschampa HJ, et al. Brain tumors: full- and half-dose contrast-enhanced MR imaging at 3.0 T compared with 1.5 T--Initial Experience. Radiology 2005;237:1014-9. [PubMed]
- Katayama H, Yamaguchi K, Kozuka T, et al. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990;175:621-8. [PubMed]
- Dillman JR, Ellis JH, Cohan RH, et al. Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am J Roentgenol 2007;189:1533-8. [PubMed]
- Neil-Dwyer JG, Ludman CN, Schaverien M, et al. Magnetic resonance angiography in preoperative planning of deep inferior epigastric artery perforator flaps. J Plast Reconstr Aesthet Surg 2009;62:1661-5. [PubMed]
- Hartung MP, Grist TM, Francois CJ. Magnetic resonance angiography: current status and future directions. J Cardiovasc Magn Reson 2011;13:19. [PubMed]
- Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-Associated Plaques: A New, Distinctive Clinical Entity. JAMA Dermatol 2015;151:316-9. [PubMed]
- Edwards BJ, Laumann AE, Nardone B, et al. Advancing pharmacovigilance through academic-legal collaboration: the case of gadolinium-based contrast agents and nephrogenic systemic fibrosis-a Research on Adverse Drug Events and Reports (RADAR) report. Br J Radiol 2014;87:20140307. [PubMed]
- Perazella MA, Rodby RA. Gadolinium-induced nephrogenic systemic fibrosis in patients with kidney disease. Am J Med 2007;120:561-2. [PubMed]
- Hedley AJ, Molan MP, Hare DL, et al. Nephrogenic systemic fibrosis associated with gadolinium-containing contrast media administration in patients with reduced glomerular filtration rate. Australas Radiol 2007;51:300-8. [PubMed]
- Internal Agency for Research on Cancer. GLOBOCAN 2008: cancer incidence and mortality worldwide. Available online: http://www.iarc.fr/en/media-centre/iarcnews/2010/globocan2008.php, accessed on 1, February, 2015.
- Azuma R, Morimoto Y, Masumoto K, et al. Detection of skin perforators by indocyanine green fluorescence nearly infrared angiography. Plast Reconstr Surg 2008;122:1062-7. [PubMed]
- Rozen WM, Chubb D, Ashton MW, et al. Achieving high quality 3D computed tomographic angiography (CTA) images for preoperative perforator imaging: now easily accessible using freely available software. J Plast Reconstr Aesthet Surg 2011;64:e84-6. [PubMed]


