
Prognostic value of different amounts of cancer stem cells in different molecular subtypes of breast cancer
Introduction
Cancer stem cells (CSCs) refer to a very small amount of cancer cell subgroups with stem cell properties in tumor tissues, which have the potential of multi-directional differentiation and the capacity of unlimited proliferation and self-renewal. They are capable of dividing asymmetrically to produce one stem cell, which enables the capacity for self-renewal, and one progenitor cell, which allows them to produce phenotypically diverse cancer cells that constitute tumors. And the existence of breast cancer stem cells (BCSCs) in malignant breast tumors also has been demonstrated in many previous studies (1-3).
In recent years, scholars at home and abroad believe that breast cancer tissues contain a group of self-replication and differentiation breast cancer cells with characteristics of stem cell-like tumor origin and invasive features, which are insensitive to chemotherapy or radiotherapy and also account for the tumor recurrence or metastasis after the breast cancer surgery (4-6). These CSC undergo both self-renewal and differentiation, and have a high tumorigenic potential. The pluripotent nature of CSC may be responsible for driving tumor initiation, progression and the development of solid mammary tumors that comprises of the cell diversity seen in breast cancer (7). These stem cells exhibit a range of phenotypes, including CD44+CD24- and CD44+CD24-/dim. They can escape the effects of chemotherapy or radiation therapy, therefore they possess specific characteristics, such as anti-tumor-drug and radiation resistance (8). Given their tumorigenic properties and increased resistance to therapy, BCSCs have been implicated in therapeutic relapse. The BCSCs that survived the selective pressure exerted by therapy may pass on reduced sensitivity to their progeny, promoting the appearance of clinical resistance and allowing evolution of a more aggressive tumor over time.
Currently, breast cancer can be classified into five molecular subtypes, which differ not only in postoperative recurrence and metastasis rates, but also in their sensitivity to chemotherapy (9). It was demonstrated that the presence of cancer stem cells in breast tumors is particularly associated with specific subtypes (10-12). However, BCSC population within a triple [estrogen receptor (ER), progesterone receptor (PR) and HER-2] negative cell line can be depleted by radiotherapy, supporting the hypothesis that resistance in not an intrinsic feature of BCSCs.
Moreover, breast cancer progression to metastatic disease may be initiated by invasive CSC, which is supported by studies showing that tumors rich in CSC have a significantly worse clinical outcome (13). Indeed, it is thought by a growing number of oncologists that it is only by controlling the CSC component, that cancer can be truly cured (14,15). Therefore, although CSC comprise only a small proportion of cells. within a tumor, they should be considered an important target for therapeutic intervention. Clinical analyses of BCSCs in breast tumors also have found a correlation between the proportion of BCSCs and poor prognosis. Therefore, new therapies that specifically target BCSCs are an urgent need. With regards to this, this article intends to determine the amount of cancer stem cells within breast cancer tissues of different subtypes, in order to explore the relations of the amount of such stem cells with molecular subtypes of breast cancer and its clinical implication.
Materials and methods
Molecular subtypes of breast cancer and corresponding clinical data
The specimens of breast cancer tissues in our hospital from January 2010 to June 2011 were collected, which were subject to pathological confirmation via mass fine-needle aspiration preoperatively combined with immunohistochemical determination of molecular subtypes of breast cancer and assigned into five groups based on different molecular subtypes, group A (luminal A type): ER (+)/PR (+), HER-2 (-); B (luminal B type): ER (+)/PR (+), HER-2 (+); C (HER-2+ type), D (basal-like type): ER (-), PR (-), HER-2 (-), CK5/CK14 (+) and E (normal-like type): ER (-), PR (-), HER-2 (-), CK5/CK14 (-). HER-2 (+) was verified by the fluorescence in situ hybridization (FISH) experiment. Each group contained 20 female cases who had not received radiotherapy or chemotherapy preoperatively; no significant statistical differences were found among the five groups in age, lymph node metastasis or histological grade.
Reagents and instruments
The reagents and instruments used in this study mainly included serum-free DMEM-F12 and fetal bovine serum purchased from the HyClone Corporation; B27 Supplement, basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) purchased from the Gibco Corporation; LeiTCS SP5 laser confocal microscope; ultraclean working platform purchased from the AIRTECH Corporation.
Cancer stem cell isolation and counting
Breast cancer tissue specimens of 2 cm × 2 cm in size were obtained, ground into a muddy state using a cell strainer, rinsed with D-Hanks solution, filtered, centrifuged and digested with trypsinase to prepare a single cell suspension, placed in DMEM medium containing 10% fetal calf serum and incubated at 37 °C in a 5% CO2 constant-temperature incubator. After centrifugation and replacement of fluid, cells growing logarithmically were observed for growth, inoculated to serum-free DMEM-F12 culture medium containing growth factors (1:50 B27, 20 ng/mL EGF, 20 ng/mL bFGF) when some of them grew in colony as spheres and incubated at 37 °C in a 5% CO2 constant-temperature incubator, fluid replaced once every 3 d and continuing when cell spheres grew stably.
Cell “pellets” were prepared into a single cell suspension, placed into serum-free culture solution diluted to 0.5 × 105 cells/mL and inoculated to a 96-well plate, 20 µL in each well and about 1,000 cells/well. After cell cultivation for 6 d, laser confocal microscopy was applied to calculate the number of cell spheres in each well.
Statistical analysis
SPSS16.0 statistical software was used for data analysis and experimental results were expressed as mean±SD. The collected data of different groups were tested by the F test and inter-group comparisons were undertaken by the q test.P<0.05 was regarded as statistically significant.
Results
The process of breast cancer stem cell formation
Breast cancer stem cells grew in colony in the process of cultivation, as non-adherent “cell spheres”. Cell spheres became stable on the 7th day, which is the optimal time for counting. Nonetheless, mature cells could not grow in serum-free DMEM-F12 medium (Figure 1).
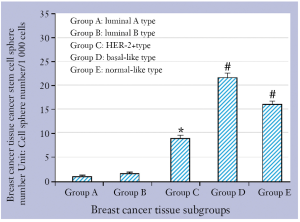
Breast cancer stem cell sphere number in the five groups
The numbers of cancer stem cell spheres in group A and B were (1.1±0.2)/1,000 cells and (1.3±0.1)/1,000 cells, respectively; cancer-containing stem cell spheres were the lowest in the two groups of breast cancer tissues and there was no significant statistical difference between the two groups (P>0.05). The number of cancer stem cell spheres in group C was (8.6±1.0)/1,000 cells, which was significantly higher than that in group A and B (both P<0.05). The numbers of cancer stem cell spheres in group D and E were (22.4±1.2)/1,000 cells and (17.7±2.0)/1,000 cells, respectively, and this was statistically similar (P>0.05) but either was significantly higher than that in the other three groups (all P<0.05) (Figure 2).

Discussion
The incidence of breast cancer has risen to the second place among female cancers. In 2003, AI Hajj and Wicha et al. (16) for the first time isolated CD44+/CD24-/low cancer cell lines with characteristics of self-renewal and differentiation potential from human primary breast cancer and breast cancer metastatic pleural exudate. Less than 200 of such cells were injected into mice to induce transplantable tumors with parental heterogeneity, confirming the presence of breast cancer stem cells in breast cancer tissues. Foreign scholars succeeded in isolating cancer stem cells using different breast cancer cell surface markers (17-20). Mylona et al. (21) have found that the increased ratio of CD44+/CD24-/low cells has the trend of increasing the disease-free survival rate and is also associated with lymph node metastasis and disease staging. There are several kinds of methods for sorting breast cancer stem cells: SP method, cell colony formation method, flow cytometry and immunomagnetic beads sorting. In this experiment, the cell colony formation method was applied to sort breast stem “cell spheres”, which could grow in serum-free DMEM-F12 medium and was the most stable on the 7th day, while non-cancer-containing stem cells could not grow as “spheres”. The reason lies in: breast stem cells have the capacity of self-replication and division, the majority of which differentiate into cancer cells and the minority of which continue to divide into cancer stem cells. It still requires further research how cancer stem cells differentiate into different cells.
Research conducted by Carey et al. (22) on breast cancer molecular subtypes found that luminal A type accounted for 51.4%, luminal B type 15.5%, HER-2+ type 6.7%, basal-like type 20.2% and normal-like type 6.3%. This study found that breast cancer masses of luminal A/B type were small and sensitive to chemotherapy, had low histological grade and good prognosis, and seldom developed lymph node or hematogenous metastasis; patients with HER-2+ type and basal-like type tumors had the poorest prognosis, highest 5-year recurrence rates and shortest survival time (23). Yu Chao et al. (24) have found that atypia of basal-like type breast cancer cells is great, which lack glandular structure and have high histological grade, suggesting strong tumor invasiveness, poor prognosis and a liability to develop lymph node or hematogenous metastasis. Normal-like type subgroups express genes of adipose cell and other non-epithelial cell origin; in addition, the subgroup highly expresses basal epithelial cell genes, while breast luminal epithelial gene expression is low. The subgroup is largely distributed in the basal-like type and non-sensitive to chemotherapy, so its prognosis is poor (25). It was found in this study that the amount of cancer stem cells is the lowest in breast cancer tissues of luminal A/B type, significantly lower than that in HER-2+ type, basal-like type and normal-like type, which is consistent with the clinical and pathological features of breast cancer of different molecular subtypes. Insensitivity of cancer stem cells to chemotherapy or radiotherapy primarily accounts for the failure of tumor chemotherapy (26), so sensitivity of breast cancer of different molecular subtypes to chemotherapy also differs. This is also one of the major causes of recurrences or metastases after the operation on breast cancer of different molecular subtypes.
In conclusion, breast cancer stem cell amount can predict recurrences or metastases after the breast cancer operation, which is expected to be regarded as one of the prognostic indices of breast cancer. Research on elevating the sensitivity of cancer stem cells to chemotherapy or radiotherapy still await in-depth investigation, hoping to provide a new way for treating postoperative recurrences or metastases after the breast cancer operation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [PubMed]
- Ginestier C, Wicinski J, Cervera N. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle 2009;8:3297-302. [PubMed]
- Wright MH, Calcagno AM, Salcido CD, et al. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res 2008;10:R10. [PubMed]
- Zhang M, Rosen JM. Stem cells in the etiology and treatment of cancer. Curr Opin Genet Dev 2006;16:60-4. [PubMed]
- Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 2006;98:1777-85. [PubMed]
- Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008;100:672-9. [PubMed]
- Oliveira LR, Jeffrey SS, Ribeiro-Silva A. Stem cells in human breast cancer. Histol Histopathol 2010;25:371-85. [PubMed]
- Dave B, Chang J. Treatment resistance in stem cells and breast cancer. J Mammary Gland Biol Neoplasia 2009;14:79-82. [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [PubMed]
- Honeth G, Bendahl PO, Ringnér M, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res 2008;10:R53. [PubMed]
- Storci G, Sansone P, Trere D, et al. The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J Pathol 2008;214:25-37. [PubMed]
- Korkaya H, Paulson A, Iovino F, et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120-30. [PubMed]
- Shipitsin M, Polyak K. The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab Invest 2008;88:459-63. [PubMed]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [PubMed]
- Li M, Knight DA, Smyth MJ, et al. Sensitivity of a novel model of mammary cancer stem cell-like cells to TNF-related death pathways. Cancer Immunol Immunother 2012;61:1255-68. [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [PubMed]
- Vassilopoulos A, Wang RH, Petrovas C, et al. Identification and characterization of cancer initiating cells from BRCA1 related mammary tumors using markers for normal mammary stem cells. Int J Biol Sci 2008;4:133-42. [PubMed]
- Liu S, Ginestier C, Charafe-Jauffret E, et al. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA 2008;105:1680-5. [PubMed]
- Bussolati B, Grange C, Sapino A, et al. Endothelial cell differentiation of human breast tumour stem/progenitor cells. J Cell Mol Med 2009;13:309-19. [PubMed]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [PubMed]
- Mylona E, Giannopoulou I, Fasomytakis E, et al. The clinicopathologic and prognostic significance of CD44+/CD24(-/low) and CD44-/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol 2008;39:1096-102. [PubMed]
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502. [PubMed]
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [PubMed]
- Pazaiti A, Fentiman IS. Basal phenotype breast cancer: implications for treatment and prognosis. Womens Health (Lond Engl) 2011;7:181-202. [PubMed]
- Finak G, Sadekova S, Pepin F, et al. Gene expression signatures of morphologically normal breast tissue identify basal-like tumors. Breast Cancer Res 2006;8:R58. [PubMed]
- Li Z, Liu C. The expression of multi-drug resistance gene in breast cancer stem cells and its significance. Tumor 2008;28:129-31.


