
Correlations of ALDH1 expression with molecular subtypes and ABCG2 in breast cancer
Introduction
Breast cancer is a highly heterogeneous malignant tumor, and patients with the same histological type and pathological grade may have extremely different responses and outcomes even with treatment of the identical regimen. Moreover, breast cancer is characterized by a remarkable biological heterogeneity both between and with in tumors. Although the mechanisms leading to intratumoral heterogeneity in breast cancer are not understood, a subpopulation of cancer cells, cancer stem cells (CSCs), that have some phenotypic similarities with adult tissue stem cells, has been suggested to contribute to tumour heterogeneity (1). The features of CSCs include the ability to differentiate to recreate all cell phenotypes of the parent tumor and expression of high levels of ATP-binding cassette (ABC) drug transporters,especially ATP-binding cassette sub-family G member 2 (ABCG2) (2).
These stem cells were initially isolated based on the presence of markers such as CD44, CD24, and ALDH1. In 2003, Al-Hajj and colleagues (3) isolated breast cancer cells with the phenotype of CD44+/CD24-/Low from eight breast cancer patients using flow cytometry, which were characterized by such potentials as self-renewal, multi-directional differentiation and strong oncogenicity. Thus, the strains confirmed the presence of breast cancer stem cells. Aldehyde dehydrogenase 1 (ALDH1) is a detoxifying enzyme responsible for the oxidation of intracellular aldehydes and thereby confers resistance to alkylating agents (4,5), it might protect against oxidative stress to affect the longevity of stem cells. And ALDH1 may have a role in early differentiation of stem cells as well as stem cell proliferation through its role in oxidizing retinol to retinoic acid, a modulator of cell proliferation (5,6). Moreover, its expression is associated with unfavorable tumor characteristics in breast cancer, such as high grade, absence of hormone receptor expression, positive HER2 status and the basal-like molecular subtype (6-8). Recently, ALDH1 has been identified as a reliable marker of breast cancer stem cells and its clinical significance as a prognostic indicator of breast cancer had been reported by several investigators (9). Yasuyo Ohi et al. found that TN breast cancer expressed ALDH1 more frequently than non-TN breast cancer (10).
Molecular profiling has provided biological evidence for heterogeneity of breast cancer through the identification of intrinsic subtypes. Analysis of gene expression data suggest that breast cancers can be divided into molecular subtypes which have distinct clinical features, with markedly differing prognosis and clinical outcomes. Using a panel consisting of estrogen receptor (ER), progesterone receptor (PR), HER2, CK5/6, breast cancers could be classified as two estrogen receptor (ER) positive types (luminal A and luminal B), and three ER-negative types (HER2 expressing, basal like and normal breast-like) (11-13).
In this study, the purpose was to detect the expression of ALDH1 and the association between its distribution in breast cancers of different molecular subtypes and ABCG2.
Materials and methods
Specimens and general information
A total of 179 paraffin-embedded specimens were collected from patients undergoing radical mastectomy in our hospital from September 2009 to December 2010. Each of the diagnosis of breast cancer was postoperatively confirmed by pathological examination. The patients were female, aged from 29 to 79 years with a median age of 52.3 years, including 56 premenopausal cases and 123 post-menopausal cases. Seventy patients did not receive chemotherapy before surgery, while 109 did. None of them were subject to endocrine therapy or HER2 targeted therapy preoperatively. According to the pathological diagnosis reports by Chongqing Medical University, invasive ductal carcinoma, invasive lobular carcinoma, medullary carcinoma and mucinous carcinoma, as well as other types, were identified in the 179 patients with breast cancer. An overview of the estrogen receptor (ER), progesterone receptor (PR) and HER2 (HER2 immunohistochemical staining of 3+ considered positive) (14) results is shown in Table 1 and the presence and number of lymph node metastasis in Table 1.
Full table
Classification of breast cancer molecular subtypes
The lesions were classified into five molecular subtypes based on the immunohistochemical indicators ER, PR, HER2 and CK5/6 suggested by Sorlie and Nielsen et al. (15,16): luminal A (ER and/or PR-positive and HER2-negative), luminal B (ER and/or PR-positive and Her2-positive), HER2 over-expression (ER-negative and PR-negative, HER2-positive), basal-like (ER-negative, PR-negative, HER2-negative, and CK5/6-positive) and normal breast-like (ER-negative, PR-negative, HER2-negative, and CK5/6-negative).
Reagents and experimental methods
Reagents
The ALDH1 rabbit anti-human polyclonal antibodies were purchased from ABCAM company, CK5/6 rabbit anti-human polyclonal antibodies and ABCG2 rabbit anti-human polyclonal antibodies from Beijing Boaosen Biotechnology Co., Ltd., and SP immunohistochemical staining kits and diamino benzidine (DAB) chromogenic reagent kits from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.
Immunohistochemistry method
Forty-one “triple negative” specimens (ER-negative, PR-negative and HER2-negative) were made into 4 µM paraffin sections, followed by conventional xylene dewaxing, graded ethanol hydration, and antigen repair at high temperature in citrate buffer solution (pH 6.0) for 15 min. The specimens were soaked in 3% H2O2 at room temperature for 10 min, washed three times with PBS, incubated in goat serum at 37 °C for 20 min, and stored overnight at 4 °C with dropping of CK5/6 antibodies (concentration 1:100). With drops of horseradish peroxidase labeled secondary antibodies, the sections were incubated at 37 °C for 20 min, washed three times with PBS, rinsed with DAB for colorization and then tap water, mildly restained with hematoxylin, and mounted with neutral resin.
On the other hand, 179 specimens collected from breast cancer patients were also made into 4 µM sections for immunohistochemical staining following the same procedures except that ALDH1 and ABCG2 antibody solutions with a concentration of 1:100 were respectively added in lieu of CK5/6.
Determination of results
Totally 100 cells were counted in each of the ten high power fields randomly selected on every section to generate the average cell count. CK5/6 expression was located in the cytoplasm of breast cancer cells. For assessment of antibody staining, a positive status (+) was identified when positive cells ≥5%, and negative (–) when <5%. ALDH1 protein staining was brownish-yellow, mostly in the cytoplasm. The staining results were graded according to the intensity and proportion of stained cells. Cells were rated based on the presence and intensity of staining on the sections: 0 for no color, 1 for light brown, and 2 for dark brown. The proportion of stained cells was rated as 0 for 0-10%, 1 for 11-30%, 2 for 31-70%, and 3 for >71%. The subjects were classified by the sum of those two ratings, with a score of 0 being negative (–) and 1-5 being positive (+) (17) The brownish-yellow ABCG2 staining was present in the cytoplasm and cell membranes, rated based on the proportion of stained cells (1 for ≤10% positive cells, 2 for 11-50%, 3 for 51-75%, and 4 for >75%) and the staining intensity (0 for no color, 1 for pale yellow, 2 for brownish-yellow, and 3 for dark brown). The subjects were classified by the product of those ratings, with 0-3 being negative (–) and ≥4 being positive (+).
Statistical analysis
The data were analyzed in SPSS17.0 using Chi-square tests for enumeration contents. P<0.05 was considered significantly different.
Results
Expression of CK5/6 in triple-negative breast cancer cells and distribution of molecular subtypes
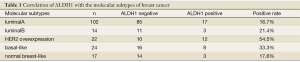
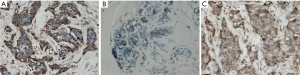
CK5/6 expression was present in the cytoplasm of breast cancer cells in brownish-yellow (Figure 1A). In the 41 triple-negative cases, 24 were positive for CK5/6 expression (58.5%) and 17 negative (41.5%). The distribution of molecular subtypes in the 179 cases was: 102 cases of luminal type A (57.0%), 14 cases of luminal type B (7.8%), 22 cases of HER2 overexpression (12.3%), 24 cases of basal-like type (13.4%) and 17 cases of normal breast-like type (9.5%).
ALDH1 expression in 179 breast cancer patients and distribution of ALDH1-positive cells in various molecular subtypes
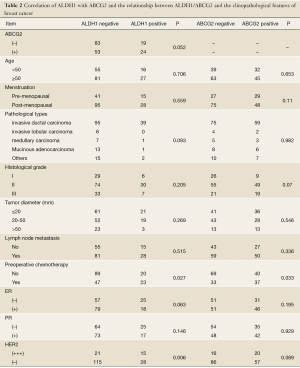
ALDH1 expression was present in the cytoplasm of breast cancer cells in brownish-yellow (Figure 1B). Out of the 179 subjects, 43 were positive for ALDH1 expression (24.0%) and 136 were negative (76.0%). Significant differences in the levels of ALDH1 expression were observed among different molecular subtypes (P=0.003). High expression levels were found in the HER2 overexpression type (54.5%) and basal-like type (33.3%) (Table 2).
Full table
Relationship between ALDH1, ABCG2 and the clinical pathological characteristics of breast cancer
ABCG2 was expressed in the cytoplasm and cell membranes and stained in brownish-yellow (Figure 1C). Of the 179 subjects, 77 were positive for ABCG2 expression (43.0%) and 102 were negative (57.0%). No statistical difference was observed in the ALDH1 expression levels between the subgroups by ABCG2, age, menstruation, pathological type, histological grade, tumor diameter, presence or absence of lymph node metastasis, or ER and PR factors (P>0.05), though there was difference between subgroups by the preoperative chemotherapy (P=0.027) and by HER2 expression (P=0.006). Meanwhile, ABCG2 was only associated with the presence of preoprative chemotherapy (P=0.033) (Table 1).
Discussion
According to the cancer stem cell theory (18), tumor tissues consist of heterogeneous cell populations. Among them, a small fraction of cells have the capacity of stem cells and are the determinant of the tumor occurrence, development, prognosis, metastasis and sensitivity to a variety of treatments, while most other cells would die after a limited number of proliferation cycles and thus are unable to become tumors.
ALDH1 family contains a series of NADP+ dependent enzymes that catalyze the intracellular oxidation of acetaldehyde to acetic acid. Essential to the growth and differentiation of normal stem cells in hematopoietic tissue or others, those substances are highly active in stem cells and progenitor cells. In 2007, Ginestier et al. (19) used ALDH1 to isolate and identify stem cells from breast cancer tissues, finding that ALDH1-positive cells accounted for only 5% of all breast cancer cells, though they were capable of self-renewal and multilineage differentiation. They also found in the tumor formation study that merely 500 ALDH1-positive cells were sufficient to generate a new tumor, a mission impossible with as many as 50,000 negative ones, suggesting high tumorigenicity of the positive cells. In addition, they found that the ALDH1-positive status was positively correlated with a high histological grade, HER2 overexpression, and the positive status of ER and PR, but not associated with the patient age, tumor size or lymph node metastasis. The 5-year overall survival analysis showed that the ALDH1 positive status was linked with poor outcomes in both groups. In a immunohistochemical study with 203 breast cancer patients, Morimoto and colleagues (20) found that the ALDH1 positive status was positively correlated with ER-, PR-, HER2+, Ki67+ and TOP2A+, independent of age, menopause, tumor size, lymph node metastasis and histological grading.
In the present study, the ALDH1 expression was linked with preoperative chemotherapy with significant difference between subgroups by the two components, regardless of age, menopause, pathological type, histological grade, tumor size, lymph node metastasis and PR, as there was no significant difference between subgroups by the latter factors. Similar to the findings of the above two studies, that result could be one of the explanations for varying clinical outcomes after a single regimen in breast cancer patients with identical clinical manifestations. Of the 179 subjects in this study, only 43 had expression of ALDH1, accounting for a low proportion of 24.0%. That was also consistent with the relatively small fraction of stem cells in the tumor tissue.
To explain the heterogeneity of breast cancer, Sorlie and coworkers (15) employed CDNA microarray analysis of breast cancer genes and found large differences in gene expression between different types of breast cancer. They divided the tumors into ER+ and ER- subgroups based on the cluster analysis of gene expression, of which the ER+ subgroup was further divided into luminal A and luminal B types and the ER- subgroup HER2 overexpression, basal-like and normal breast-like types. The outcomes differed dramatically between different molecular subtypes, with those of HER2 overexpression and basal-like types being the poorest. Dontu et al. (21) proposed a new cancer stem cell classification hypothesis based on the tumor stem cell origin theory. He suggested that the stem cells in breast cancer evolved into three different populations in the process of occurrence and development: (I) those derived from ER- stem cells that contained few ER+ cells, which were unresponsive to therapeutic interventions and thus associated with poor outcomes; (II) those derived from ER- stem cells but gradually converting into ER+ cells because of variation during the development of tumor, which were initially sensitive to estrogen treatment but later more and more resistant with the continued proliferation of ER- cells or downregulated ER+ expression due to new mutations; and (III) those derived from ER+ stem cells and progenitor cells, which were sensitive to treatments and associated with good outcomes. Concurring with the subtyping of breast cancer, the hypothesis explained the relatively poor outcomes in conditions of HER2 overexpression and basal-like types from the perspective of stem cell theory.
Morimoto et al. (20) noted that expression of ALDH1 was the highest in the HER2 overexpression type, followed by the triple-negative one. Nalwoga et al. (22) noted that ALDH1 was highly expressed in basal-like breast cancer. Charafe-Jauffret et al. (23) and Park et al. (24) also found that ALDH1-positive stem cells were linked with basal-like and HER2-overexpression breast cancers. Zhou et al. (17) found that ALDH1 expression was positively correlated with HER2 overexpression. Multivariate prognostic analysis suggested that, like HER2 overexpression, ALDH1 was another risk factor of poor outcomes. The HER2 gene was closely related to the self-renewal and self-regulation of tumor stem cells. It was noted the ALDH1 expression levels differed significantly between subtypes, being the highest in the HER2 overexpression and basal-like types at 54.5% and 33.3%, respectively. The present study was consistent with the above findings.
Dean (25) proposed a hypothesis for drug-resistant tumor stem cells that the high expression of multidrug-resistance proteins in the cells underlay the failure of treatment, relapse and metastasis by inducing resistance of the entire tumor cell populations against chemotherapeutic drugs. ABCG2, a member of the multidrug-resistance proteins, protected stem cells from damage by cytotoxic agents by enabling the pump-out of fluorescent dye HoeCHst33342 from the stem cells (26,27) Prud’homme et al. (28) noted a high expression of ALDH1 and ABCG2 in the breast cancer cell microspheres formed after drug intervention. Dong and coworkers (29) isolated chemotherapeutic microspheres from breast cancer patients undergoing neoadjuvant therapy, and found a high expression of ALDH1 in them. The present study also suggested a significant difference in the expression of both indicators with and without preoperative chemotherapy. Although the expression of ALDH1 did not differ from that of ABCG2, it was higher in the ABCG2-positive group (31.2%) than in the negative one (16.8%). Further research would be needed to identify ABCG2 as a contributor to the chemoresistance in ALDH1-positive breast cancer. As shown in another study, tumor stem cells stayed in the relatively static G0 or G1 phase, while the surrounding cells (progenitor cells) in the active S, M or G2 phase with rapid proliferation (30,31) However, most existing therapies were only effective against differentiated tumor cells or those in the active proliferation cycle, allowing stem cells in the slow phases to evade cytotoxic engagement and become a source of future recurrence.
The biological functions and acting mechanisms of ALDH1 remained unclear. With a high expression level of ALDH1, HER2 overexpression, basal-like and ABCG2-positive types of breast cancer were associated with poor outcomes and treatment resistance. A targeted, effective treatment against ALDH1 could only be achieved with further research on ALDH1 for individual therapies on different molecular subtypes and minimized treatment resistance.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Badve S, Nakshatri H. Breast-cancer stem cells-beyond semantics. Lancet Oncol 2012;13:e43-8. [PubMed]
- Prud’homme GJ, Glinka Y, Toulina A, et al. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS One 2010;5:e13831. [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [PubMed]
- Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 2009;15:4234-41. [PubMed]
- Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res 2009;69:3382-9. [PubMed]
- Mieog JS, de Kruijf EM, Bastiaannet E, et al. Age determines the prognostic role of the cancer stem cell marker aldehyde dehydrogenase-1 in breast cancer. BMC Cancer 2012;12:42. [PubMed]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [PubMed]
- Nalwoga H, Arnes JB, Wabinga H, et al. Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br J Cancer 2010;102:369-75. [PubMed]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [PubMed]
- Ohi Y, Umekita Y, Yoshioka T, et al. Aldehyde dehydrogenase 1 expression predicts poor prognosis in triple-negative breast cancer. Histopathology 2011;59:776-80. [PubMed]
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502. [PubMed]
- Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418-23. [PubMed]
- Salhia B, Tapia C, Ishak EA, et al. Molecular subtype analysis determines the association of advanced breast cancer in Egypt with favorable biology. BMC Womens Health 2011;11:44. [PubMed]
- van de Rijn M, Perou CM, Tibshirani R, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 2002;161:1991-6. [PubMed]
- Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418-23. [PubMed]
- Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367-74. [PubMed]
- Zhou L, Yu P, Wang J, et al. Expression of aldehyde dehydrogenase 1 in breast cancer and its clinical sign ificance. Tum 2009;29:663-7.
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [PubMed]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [PubMed]
- Morimoto K, Kim SJ, Tanei T, et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci 2009;100:1062-8. [PubMed]
- Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab 2004;15:193-7. [PubMed]
- Nalwoga H, Arnes JB, Wabinga H, et al. Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br J Cancer 2010;102:369-75. [PubMed]
- Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 2009;69:1302-13. [PubMed]
- Park SY, Lee HE, Li H, et al. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res 2010;16:876-87. [PubMed]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5:275-84. [PubMed]
- Raaijmakers MH, de Grouw EP, Heuver LH, et al. Breast cancer resistance protein in drug resistance of primitive CD34+38- cells in acute myeloid leukemia. Clin Cancer Res 2005;11:2436-44. [PubMed]
- Zhou S, Morris JJ, Barnes Y, et al. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A 2002;99:12339-44. [PubMed]
- Prud’homme GJ, Glinka Y, Toulina A, et al. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS One 2010;5:e13831. [PubMed]
- Dong H, Wu C, Chen Y. Isolation,culture and identification of microspheres in breast cancer stem cells after neoadjuvant chemotherapy. Journal of Jilin University 2011;1:163-7. (Medicine Edition).
- Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood 2003;101:3142-9. [PubMed]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755-68. [PubMed]


