Oncological safety of prophylactic breast surgery: skin-sparing and nipple-sparing versus total mastectomy
Introduction
BRCA1/2 mutation carriers have a cumulative lifetime breast cancer risk of 55-85% by the age of 70 (1-5). As an alternative to surveillance, BRCA1 and BRCA2 mutation carriers and other women with a high breast cancer risk may choose to undergo bilateral prophylactic mastectomy, reducing breast cancer risks by 90-100% after 3-13 years of follow-up (6-10). The prophylactic character of the bilateral mastectomy emphasizes the importance of a natural aesthetic outcome (11), which can be achieved by various immediate autologous and implant breast reconstruction techniques. Instead of the conventional total mastectomy, to allow for an immediate breast reconstruction and to achieve a natural aesthetic outcome so-called conservative mastectomies are increasingly performed for risk reduction. In conservative mastectomies, all breast glandular tissue is removed while leaving the skin envelope and, if spared, the nipple-areola complex (NAC) in situ [skin-sparing mastectomy (SSM) and nipple-sparing mastectomy (NSM), respectively].
Safety of conservative mastectomies in women at high breast cancer risk is subject to an ongoing debate. The presumed oncological risk of the conservative technique lies in potential remaining breast glandular tissue with the skin flap and, if spared, with the NAC. Smaller incisions that are tailored to individual reconstruction wishes, however, may result in a technically difficult surgical approach. Therefore, the oncological safety of the conservative mastectomy remains a challenge for the oncological surgeon. We present a case of primary breast cancer developed after prophylactic conservative mastectomy. Further, we provide a review of the literature on the oncological safety of prophylactic conservative mastectomies.
Case: a 43-year-old woman with primary breast cancer in the prophylactic mastectomy scar
In 2011, a 43-year-old woman presented a lesion clinically suspicious of breast cancer. In 1982, at the age of 15, she had been successfully treated for stage IIa Hodgkin’s disease in her neck and mediastinum with 40 Gy mantle field radiation. After 10 years there were no signs of recurrence and she was discharged from follow-up.
In 1998, a mammography—performed because of a wish for breast reduction—revealed suspect microcalcifications in the left breast. The suspect lesion was excised by upper outer quadrantectomy. Pathological examination of the lumpectomy specimen showed grade 2 ductal carcinoma in situ. No adjuvant radiotherapy was administered due to the history of mantle field radiation. Initially, physicians and patient agreed to frequent radiological screening instead of a completing mastectomy. However, after several additional diagnostic procedures due to suspect lesions of the left breast, in 2001, the patient chose to undergo a SSM and immediate implant reconstruction. In 2003, this was followed by a prophylactic SSM of the right breast and bilateral implant reconstruction. In both cases, histologic investigation showed no (in situ) malignancy.
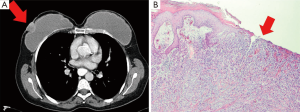
In 2011, she returned with an ulcerous lesion in the right mastectomy scar. On CT-scan a superficial tumor of 21×27 mm2 was seen (Figure 1A). Ultrasonography of the axilla did not show pathological lymph nodes. A wide local excision with axillary lymph node dissection was performed and the implants were removed. Histological examination of the excised specimen showed an invasive ductal carcinoma with a diameter of 2.4 cm, Bloom Richardson grade 3, estrogen receptor (ER) positive, progesterone receptor (PR) and human epithelial growth factor-2 receptor (HER2 receptor) negative (Figure 1B). Adjacent to the tumor, normal glandular breast tissue was found. One out of eight dissected axillary nodes showed a metastasis. According to our national protocol, she received adjuvant chemotherapy, hormonal therapy and re-irradiation with hyperthermia of the chest wall. At the time of writing the patient is alive without breast cancer recurrence.
Surgical techniques of conservative mastectomies: SSM and NSM
Examples of conservative mastectomies include SSM and NSM. In SSM, a periareolar incision is used with caudal or lateral extension if necessary (“racquet” incision). The skin envelope is created by subcutaneously excising the breast glandular tissue while preserving a thin subcutaneous layer to support skin vascularization. Nipple-papilla and surrounding pigmented areola (NAC) are removed. In NSM, the skin envelope is created through a semicircular periareolar or an inframammary incision. The NAC is dissected as thin as possible by macroscopically removing all breast glandular tissue while preserving vascularization. The nipple-papilla is “cored” by inverting it and excising residual breast glandular tissue. The NAC is then left in situ adherent to the skin envelope. A breast reconstruction is performed during the same procedure. The oncological safety of SSM in the prophylactic setting is generally acknowledged, whereas safety of NSM is still subject to debate.
In the last two decades of the past century it was common to perform a so-called subcutaneous mastectomy. Although subcutaneous mastectomy encompassed a skin- and nipple-sparing technique as well, it is likely that this was not comparable to current NSM and SSM techniques. A description of the ‘state of the art’ subcutaneous mastectomy in 1983 mentions that a plaque of one centimeter of breast glandular tissue should be left in situ with the areola (12). In contrast, current NSM and SSM techniques aim for skin flaps <5 mm and NACs of 2-3 mm thickness (13).
Breast glandular tissue or terminal duct lobular units (TDLUs): residuals after mastectomy
The hazard of remaining breast glandular tissue after mastectomy for development or recurrence of breast cancer has been a recurring subject to debate since more than half of a century. Anatomically the NAC is a continuation of the mammary gland and therefore should be removed when pursuing a complete mastectomy. Therefore, especially sparing of nipple and areola in NSM has been a controversial topic. However, the growing ability of more specifically identifying women at high breast cancer risk and the consequently increasing interest in prophylactic mastectomies has revived the discussion. Breast cancer is thought to originate in TDLUs, defined as a terminal duct combined with an associated lobule (14-16). Consequently, theoretically any remaining TDLUs may represent a lifelong potential breast cancer hazard. To estimate the remaining risk after prophylactic mastectomy, some authors have studied whether TDLUs are left in situ. Several others have simply examined the presence of remaining ductal or lobular structures or more non-specifically the presence of glandular tissue.
Residual breast glandular tissue after total mastectomies
The first study to investigate the amount of glandular tissue left in situ after a conventional total mastectomy was already in 1940 by Hicken et al. (17). The authors had been triggered by two cases of women who developed breast cancer and mastitis of residual axillary breast tissue 15 and 10 years, respectively, after an ipsilateral mastectomy for a benign indication. Mammographies of 385 breasts using intraductal contrast showed that mammary ducts frequently extend beyond regular mastectomy resection planes. In 95%, mammary ducts extended into the axillary fossa, in 15% downward into the epigastric region, in 2% beyond the lateral limits of the latissimus dorsi muscle and in two cases even past the midsternal line to the contralateral side (17). A histological analysis of 17 total mastectomies was performed in the same study by preoperatively injecting methylene blue dye into the ducts of the nipple-papilla. Any resection plane that colored blue during surgery meant that ducts had been cut and the resection site was defined as ‘irradical’ (17). Results showed that breast glandular tissue had been excised irradically underneath the skin flap in 94% of cases, in 12% the axillary tail had been removed irradically, in 23% the ducts had been cut in the sternal region and in 11% in the epigastric region (17). The authors therefore concluded that, even when it is intended to perform a total mastectomy, it is seldom accomplished (17).
In 1991, a small study was performed in ten total mastectomies in five women (18). Frozen sections of skin flaps, pectoral muscle and axillary tail were examined. Similar to the results of Hicken, residual breast glandular tissue was found in caudal skin flaps, the axillary tail and even in the pectoral fascia (18). Another small study separately resected specimens specifically of the inframammary fold (IMF) and encountered small amounts of residual breast tissue in 13/24 IMF specimens (with breast glandular tissue volume/IMF specimen volume rates of 0.04%) (19).
In 2013, Griepsma et al. studied the superficial dissection planes of 206–mostly total–mastectomy specimens (20). Per mastectomy 36 biopsies were obtained from standardized locations of the subcutaneously dissected part of the total mastectomy specimens. In 76% of mastectomies, one or more biopsies contained breast glandular tissue at the resection plane. Areas of predilection were the lower outer quadrant (15% positive biopsies) and halfway the subcutaneous dissection plane between the peripheral pectoral muscle margin and central skin margin (12% positive biopsies) (20).
Residual breast glandular tissue after conservative mastectomy: SSM and NSM
Three decades after the first report on total mastectomies by Hicken et al., Goldman and Goldwyn picked up on the issue of conservative prophylactic mastectomy by performing 12 subcutaneous (skin- and nipple-sparing) mastectomies in six cadavers through an inframammary incision (21). Biopsies of post-mastectomy skin flaps, resection planes and any fibrous or adipose tissue remaining elsewhere showed residual breast glandular tissue after 83% of mastectomies (21). In all cases even, residual breast glandular tissue was found behind the spared NAC. However, the authors do not describe which biopsy sites were positive for breast glandular tissue, nor the surgical technique used for dissection of the NAC (21).
Aiming to investigate the potential value of NSM in the treatment of lobular carcinoma in situ (LCIS), Rosen and Tench (22) vertically sectioned 101 nipples in conventional mastectomies performed for breast cancer. In 17% of the nipples lobules were found and in 13% (in situ) carcinoma was encountered. The authors propose that “coring” of the nipple-papilla in NSM, which had been described before (23), is necessary to remove as much glandular tissue as possible. The NAC was further examined in 1993 (24). By inverting the projected center of the NAC—the nipple-papilla—and grossly removing all glandular tissue inside the papilla, the nipple was cored. Despite nipple-coring the authors did encounter mammary ducts in the areolar dermis (24).
In 1991, Barton et al. compared 27 conservative mastectomies with 28 modified radical mastectomies (25). Post-mastectomy biopsies were taken at the inframammary fold, parasternal region, infraclavicular chest wall, latissimus dorsi muscle border, anterior lower axilla and skin flaps. The NAC was not examined. No differences were found between the number of biopsies containing residual breast glandular tissue after conservative mastectomy (22%) and after total mastectomy (21%) (25). After conservative mastectomy, most positive biopsies (50%) originated in the skin flap. In contrary, after total mastectomy, most positive biopsies (38%) originated at the latissimus dorsi border (25).
The skin flap after conservative mastectomy was further examined in 1998 (26). The authors removed 114 small (0.5×2.0 cm2) strips of skin from the remaining skin flap in 32 patients for complete histological examination. In none of the strips ductal breast tissue was encountered (26), however, regarding the size of the strips, this negative finding may be due to a sampling error. Somewhat larger skin flaps have been examined in a more recent study (27). In 66 SSMs, skin specimens that had been removed additionally to the SSM specimen to facilitate reconstruction were examined for residual glandular tissue. Skin specimens had a mean volume of 93.9 cm3 and in specimens of only four patients (6%) residual breast tissue was found (27). However, since only a minimum of three sites per skin specimen was analyzed, again in this study a sampling error cannot be ruled out. A study of 168 SSMs for therapeutic indication analyzed the superficial margin to the dermis just above the tumor that would have been left in situ otherwise. In contrast with the two studies described above, in 89 (53%) of the cases benign breast ducts were present in the superficial margin specimen (28).
Residual TDLUs after conservative mastectomy: SSM and NSM
Several studies have more specifically studied whether TDLUs remain after SSM or NSM (22,29-31). The only study on SSM was by Torresan et al. in 2005 (32). In 42 total mastectomies, they resected the skin flap that would have been left in situ if it were a SSM and submitted 80 slides per skin specimen for examination. In contrary to the two studies mentioned earlier, they found TDLUs in 60% of the skin flaps (32). The risk of finding TDLUs strongly increased for skin flaps thicker than 5 mm (32).
The other five studies focus on NSM. Stolier et al. examined the nipple-papilla for presence of TDLUs in 2008 (29). During mastectomies, 32 nipple-papillas were transected at the junction of papilla and areola. Nipple-papilla’s were sectioned, entirely embedded and examined microscopically for presence of TDLUs. Only in three out of 32 nipple-papilla TDLUs were found. Therefore, it was concluded that TDLUs are scarce in the nipple-papilla (29). Reynolds et al. collected 62 mastectomy specimens from 33 BRCA1/2 mutation carriers and excised the NAC for histologic evaluation (30). In 24% of the NACs, TDLUs were found; only 8% was located in the papilla (30). Similarly, Kryvenko et al. studied 105 NACs from mastectomy specimens (31). Sixty-five NACs were entirely embedded for examination of presence of TDLUs; of 40 NACs only one vertical section was examined. TDLUs were found in 26% of NACs but most frequently were located in the papilla (31)—in contrast to the results of Reynolds and Stolier (29,30). It has been suggested that an areola-sparing mastectomy rather than a NAC-sparing mastectomy should be performed for risk reduction. Removing the nipple-papilla might further reduce any remaining breast cancer risk. However, this is not supported by the abovementioned studies since two of the three show a higher incidence of TDLUs in the areola versus the nipple-papilla.
Recently, our own group compared presence and numbers of TDLUs between skin flap and NAC (33). In 105 total mastectomies, the NAC and an adjacent skin-island were dissected as if an NSM was performed, and the papilla was cored. TDLUs were found in 61% of the NACs vs. 24% of the skin islands (33). Also after adjustment for volume of the excised specimens, density of TDLUs was significantly higher in the NACs as compared with the skin. Further, risk factors for presence of TDLUs were younger age and parity (vs. nulliparity) (33). We concluded that NACs, as well as skin flaps might harbor a risk for developing breast cancer, albeit very small.
Oncological safety of prophylactic mastectomy: clinical studies
In addition to the histopathological studies, we assessed whether there are any oncological consequences of the residual glandular tissue. We performed a systematic PubMed search using the term “prophylactic mastectomy [Title/Abstract] OR skin-sparing mastectomy [Title/Abstract] OR nipple-sparing mastectomy [Title/Abstract] OR subcutaneous mastectomy [Title/Abstract] OR conservative mastectomy [Title/Abstract] OR risk-reducing mastectomy [Title/Abstract] AND breast cancer [Title/Abstract]”, yielding 680 titles. Titles and abstracts were checked for relevance. Reviews and case reports were excluded, as were articles that were not in English. Also excluded were: studies that focused: (I) on merely therapeutic mastectomy and/or comprised <20 prophylactic mastectomies and/or did not report clinical follow-up outcome of prophylactic mastectomies; (II) on survival benefits of contralateral prophylactic mastectomy or oophorectomy; (III) on uptake, counseling and decision-making of prophylactic surgery.
Twenty-four studies from 1976-2014 met our criteria and are summarized in Table 1. All are observational studies describing prospective or retrospective cohorts or a case-control series. In 24 studies, 7,173 mastectomies are described of which 1,392 were for therapeutic indications and which were not considered in further analysis. Most prophylactic mastectomies were performed in BRCA1/2 gene mutation carriers and other women at high breast cancer risk. Average follow-up periods range from 10.4-168 months. Most recent studies focus on NSM rather than SSM; while in older studies conservative mastectomies are defined as ‘subcutaneous mastectomy’, suggesting that the NAC is–partly–spared. However, as described above, it is likely that in subcutaneous mastectomy the NAC and skin are not dissected as thin as modern NSM or SSM techniques dictate.
As reported by the 24 studies in Table 1, grossly, 21 primary breast cancers occurred after 6,044 prophylactic mastectomies. Of these, three occurred after a total mastectomy (0.6% of all total mastectomies), 17 occurred after a conservative mastectomy (0.3% of all subcutaneous mastectomies, NSM or SSM) and for one breast cancer the prophylactic mastectomy technique was not specified. Besides, four patients presented with distant metastases with unknown primary site. Most prophylactic mastectomies included in these studies, as well as the ones in which a primary breast cancer developed, were subcutaneous mastectomies, NSM or SSM. Nonetheless, the majority of primary breast cancers did not originate near the NAC or skin flap. Of the 21 breast cancers that developed after prophylactic mastectomy, five were encountered at the chest wall, four in the axilla, (two in the axillary tail, one in an axillary lymph node, one in an unknown location), one in the outer quadrant, one in the nipple and one “above the areola” (not further specified). In nine cases the location was unclear or not reported.
The 21 loco-regional primary breast cancers correspond with an incidence of 0.7% per woman who undergoes bilateral prophylactic mastectomy (0.35% per mastectomy). Most breast cancers that developed after conservative mastectomy were found at the chest wall or in the axilla. Although the chest wall and the axilla may be at risk in total mastectomy as well, two things should be considered: First, the origin of the breast cancer may have been the skin flap, even though it was described as ‘chest wall’. Most breast implants in immediate breast reconstruction are placed underneath the pectoral muscle. Consequently, skin-flap and chest wall are in direct contact. Therefore, although we have no information on the reconstruction techniques used in these studies, it is possible that the breast cancers developing at the chest wall actually did originate in the skin flap. Second, as mentioned before, the surgical technique of SSM and NSM using small peri-areolar or inframammary incisions can be challenging. A suboptimal exposure may impede thorough removal of remaining breast glandular tissue in all quadrants and in the axillary tail.
In four cases, breast cancer presented as metastatic disease and the primary tumor site was never found. Pathological findings specific for breast cancers, the high a priori breast cancer risk of the patient and elimination of other potential first sites because of negative radiological examinations may all have led to the conclusion that the metastatic disease most probably originated from breast cancer. The possibility that the primary tumor already may have been present in the prophylactic mastectomy specimen emphasizes the importance of standardized pathological examination of the excised specimen, and—even more—thorough radiological screening by MRI before prophylactic mastectomy.
In conclusion, the incidence of primary breast cancers after prophylactic mastectomy is very low after total as well as after conservative mastectomies. However, theoretically, according to these data, approximately one out of 140 women undergoing bilateral prophylactic mastectomy for breast cancer prevention will develop a primary breast cancer over time. Oncological surgeons should be aware of this risk and may minimize it by putting extra care in dissecting all glandular tissue, especially in the axillary tail and chest wall, and by dissecting skin flaps and NAC as thin as possible. More studies are warranted that further assess long-term oncological safety. Further, it is important to more specifically study patient satisfaction after NSM and SSM and potential differences in patient expectations. Ultimately, surgeons and patients may be able to balance any remaining oncological risk against expected benefits of NSM or SSM.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- King MC, Marks JH, Mandell JB, et al. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003;302:643-6. [PubMed]
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997;336:1401-8. [PubMed]
- Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003;72:1117-30. [PubMed]
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 2009;27:5887-92. [PubMed]
- van der Kolk DM, de Bock GH, Leegte BK, et al. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat 2010;124:643-51. [PubMed]
- Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst 2001;93:1633-7. [PubMed]
- Heemskerk-Gerritsen BA, Menke-Pluijmers MB, Jager A, et al. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Ann Oncol 2013;24:2029-35. [PubMed]
- Kaas R, Verhoef S, Wesseling J, et al. Prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers: very low risk for subsequent breast cancer. Ann Surg 2010;251:488-92. [PubMed]
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004;22:1055-62. [PubMed]
- Heemskerk-Gerritsen BA, Kriege M, Seynaeve C. Association of risk-reducing surgery with cancer risks and mortality in BRCA mutation carriers. JAMA 2010;304:2695-author reply 2695-6. [PubMed]
- Bresser PJ, Seynaeve C, Van Gool AR, et al. Satisfaction with prophylactic mastectomy and breast reconstruction in genetically predisposed women. Plast Reconstr Surg 2006;117:1675-82; discussion 1683-4.
- Woods JE. Subcutaneous mastectomy: current state of the art. Ann Plast Surg 1983;11:541-50. [PubMed]
- Sacchini V, Pinotti JA, Barros AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006;203:704-14. [PubMed]
- Jensen HM. On the origin and progression of human breast cancer. Am J Obstet Gynecol 1986;154:1280-4. [PubMed]
- Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 1975;55:231-73. [PubMed]
- Parks AG. The micro-anatomy of the breast. Ann R Coll Surg Engl 1959;25:235-51. [PubMed]
- Hicken NF. Mastectomy: a clinical pathologic study demonstrating why most mastectomies result in incomplete removal of the mammary gland. Arch Surg 1940;40:6-14.
- Temple WJ, Lindsay RL, Magi E, et al. Technical considerations for prophylactic mastectomy in patients at high risk for breast cancer. Am J Surg 1991;161:413-5. [PubMed]
- Carlson GW, Grossl N, Lewis MM, et al. Preservation of the inframammary fold: what are we leaving behind? Plast Reconstr Surg 1996;98:447-50. [PubMed]
- Griepsma M, de Roy van Zuidewijn DB, Grond AJ, et al. Residual breast tissue after mastectomy: how often and where is it located? Ann Surg Oncol 2014;21:1260-6. [PubMed]
- Goldman LD, Goldwyn RM. Some anatomical considerations of subcutaneous mastectomy. Plast Reconstr Surg 1973;51:501-5. [PubMed]
- Rosen PP, Tench W. Lobules in the nipple. Frequency and significance for breast cancer treatment. Pathol Annu 1985;20:317-22. [PubMed]
- Randall P, Dabb R, Loc N. “Apple coring” the nipple in subcutaneous mastectomy. Plast Reconstr Surg 1979;64:800-3. [PubMed]
- Schnitt SJ, Goldwyn RM, Slavin SA. Mammary ducts in the areola: implications for patients undergoing reconstructive surgery of the breast. Plast Reconstr Surg 1993;92:1290-3. [PubMed]
- Barton FE Jr, English JM, Kingsley WB, et al. Glandular excision in total glandular mastectomy and modified radical mastectomy: a comparison. Plast Reconstr Surg 1991;88:389-92; discussion 393-4. [PubMed]
- Slavin SA, Schnitt SJ, Duda RB, et al. Skin-sparing mastectomy and immediate reconstruction: oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast Reconstr Surg 1998;102:49-62. [PubMed]
- Dreadin J, Sarode V, Saint-Cyr M, et al. Risk of residual breast tissue after skin-sparing mastectomy. Breast J 2012;18:248-52. [PubMed]
- Cao D, Tsangaris TN, Kouprina N, et al. The superficial margin of the skin-sparing mastectomy for breast carcinoma: factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol 2008;15:1330-40. [PubMed]
- Stolier AJ, Wang J. Terminal duct lobular units are scarce in the nipple: implications for prophylactic nipple-sparing mastectomy: terminal duct lobular units in the nipple. Ann Surg Oncol 2008;15:438-42. [PubMed]
- Reynolds C, Davidson JA, Lindor NM, et al. Prophylactic and therapeutic mastectomy in BRCA mutation carriers: can the nipple be preserved? Ann Surg Oncol 2011;18:3102-9. [PubMed]
- Kryvenko ON, Yoon JY, Chitale DA, et al. Prevalence of terminal duct lobular units and frequency of neoplastic involvement of the nipple in mastectomy. Arch Pathol Lab Med 2013;137:955-60. [PubMed]
- Torresan RZ, dos Santos CC, Okamura H, et al. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol 2005;12:1037-44. [PubMed]
- van Verschuer VM, van Deurzen CH, Westenend PJ, et al. Prophylactic nipple-sparing mastectomy leaves more terminal duct lobular units in situ as compared with skin-sparing mastectomy. Am J Surg Pathol 2014;38:706-12. [PubMed]
- de Alcantara Filho P, Capko D, Barry JM, et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 2011;18:3117-22. [PubMed]
- Arver B, Isaksson K, Atterhem H, et al. Bilateral prophylactic mastectomy in Swedish women at high risk of breast cancer: a national survey. Ann Surg 2011;253:1147-54. [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [PubMed]
- Contant CM, Menke-Pluijmers MB, Seynaeve C, et al. Clinical experience of prophylactic mastectomy followed by immediate breast reconstruction in women at hereditary risk of breast cancer (HB(O)C) or a proven BRCA1 and BRCA2 germ-line mutation. Eur J Surg Oncol 2002;28:627-32. [PubMed]
- Evans DG, Baildam AD, Anderson E, et al. Risk reducing mastectomy: outcomes in 10 European centres. J Med Genet 2009;46:254-8. [PubMed]
- Garcia-Etienne CA, Cody Iii HS. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J 2009;15:440-9. [PubMed]
- Hagen AI, Mæhle L, Vedå N, et al. Risk reducing mastectomy, breast reconstruction and patient satisfaction in Norwegian BRCA1/2 mutation carriers. Breast 2014;23:38-43. [PubMed]
- Harness JK, Vetter TS, Salibian AH. Areola and nipple-areola-sparing mastectomy for breast cancer treatment and risk reduction: report of an initial experience in a community hospital setting. Ann Surg Oncol 2011;18:917-22. [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [PubMed]
- Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011;18:1665-70. [PubMed]
- Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2001;345:159-64. [PubMed]
- Munhoz AM, Aldrighi CM, Montag E, et al. Clinical outcomes following nipple-areola-sparing mastectomy with immediate implant-based breast reconstruction: a 12-year experience with an analysis of patient and breast-related factors for complications. Breast Cancer Res Treat 2013;140:545-55. [PubMed]
- Peled AW, Irwin CS, Hwang ES, et al. Total skin-sparing mastectomy in BRCA mutation carriers. Ann Surg Oncol 2014;21:37-41. [PubMed]
- Pennisi VR. Subcutaneous mastectomy and fibrocystic disease of the breast. Clin Plast Surg 1976;3:205-16. [PubMed]
- Skytte AB, Crüger D, Gerster M, et al. Breast cancer after bilateral risk-reducing mastectomy. Clin Genet 2011;79:431-7. [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [PubMed]
- Wagner JL, Fearmonti R, Hunt KK, et al. Prospective evaluation of the nipple-areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol 2012;19:1137-44. [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [PubMed]
- Wijayanayagam A, Kumar AS, Foster RD, et al. Optimizing the total skin-sparing mastectomy. Arch Surg 2008;143:38-45; discussion 45. [PubMed]