Loss of signal in recurrent nerve neuromonitoring: causes and management
Introduction
Routine identification of recurrent laryngeal nerve (RLN) has been recommended as the gold standard of care during thyroid surgery (1). Intraoperative neuromonitoring (IONM) has been widely accepted as an adjunct to help facilitate the identification and dissection of RLN, detect nerve injury, and elucidate its mechanism to improve surgical technique. In addition, it is also helpful to predict the outcome of vocal cord function during the operation (2-5). The RLN supplies the intrinsic muscles of the larynx. One nerve axon and all the muscle fibers innervated by it are called a motor unit. Effective muscle contraction usually requires the activation of numerous motor units at once. During IONM of RLN in thyroid surgery, measures of laryngeal electromyography (EMG) amplitude may be correlated with the number of muscle fibers participating in the polarization and these might be correlated with the function of RLN (3,6). If the EMG response is unchanged after the operation as compared with the initial EMG signal, it suggests normal intraoperative RLN function and may predict postoperative normal vocal cord function. If RLN is severely injured during the operation, most nerve fibers do not transmit nerve conduction and loss of the EMG signal (LOS) will occur when stimulating the RLN or vagus nerve. True LOS at the end of operation often indicates a postoperative fixed vocal cord, and a two-stage thyroidectomy has been recommended in patients with planned bilateral thyroid operation to avoid the disaster of bilateral vocal cord palsy in some studies (3,7). However, LOS may be false owing to several potential factors, such as monitor dysfunction, malposition of endotracheal tube (ETT) electrodes, misuse of neuromuscular blocking agents (NMBAs), etc., and may lead to unnecessary 2nd operation. A reliable modality for intraoperative LOS evaluation and management would afford the surgeon real-time information that could help guide surgical procedure and planning, especially in the patient with a planned bilateral thyroid operation.
Causes of LOS
Criteria
According to the current literature and international standards guideline statement (3), LOS is defined as EMG signal degrades to <100 µV from initial satisfactory EMG with suprathreshold level of stimulation (i.e., >1 or 2 mA) (Figure 1) on dry field.
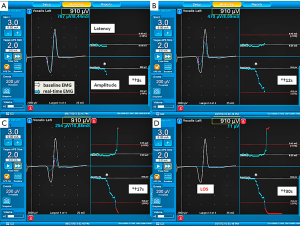
True LOS
When the RLN is injured and the elicited EMG signal <100 µV, it is called true LOS. Currently, RLN injury can be classified into two subtypes (3-5). Recent studies reported that over 70% (8-10) of injury is the type I or segmental injury that features a disrupted point or segment of nerve conduction on RLN, and this was often caused by clamping, traction, compression or thermal injury. Positive EMG signal at laryngeal entry point but negative signal at proximal point of exposed RLN or vagus nerve indicates a type I injury. We can detect the injured point by testing the RLN from laryngeal entry point to proximal area. For traction injury, the nerve is often stretched forward or compressed by the Berry’s area, and most of the injured point can be mapped in the distal 1 cm of its course (9). For type I injured nerve, surgeons might also be able to potentially correct the lesion if there is a clip, suture, vessel or fibrotic band entrapping the nerve at this point and avoid permanent RLN injury.
The other subtype is the type II or global injury that features no disrupted point of nerve conduction on the whole exposed RLN but positive response from contralateral vagus nerve stimulation. The exact mechanism of type II injury is still not well understood, but overstretching during medial retraction of the thyroid lobe was thought to be the most possible cause and the nerve might be injured at a more distal intralaryngeal focus (5).
False LOS
Normal RLN function as evidenced by the presence of a laryngeal twitch, but no EMG signal is called false LOS. It features no injured point on the exposed RLN and no response from contralateral vagus nerve stimulation. False LOS may be caused by monitoring equipment dysfunction, EMG tube malposition, or misuse of NMBAs.
Monitoring equipment dysfunction
The recording electrodes, grounding electrode and associated connections at the interface-connector box and monitor might be dislodged or displaced and should be rechecked. The use of electrocauterization might have broken the fuse.
The stimulating probe can be checked on the muscle to identify its twitch, and the monitor can be reviewed for appropriate current return.
ETT malposition
Malpositioned ETT may imply either inadequate or excessive depth relative to the vocal cords (11,12). It can occur due to excessive traction on trachea, particularly in a large goiter operation. In our experience, translaryngeal stimulation is a simple and useful method to check ETT position:
- If artifact EMG signal is elicited with 1-2 mA on thyroid and tracheal cartilage, it suggests normal functioning of monitor equipment.
- If artifact EMG signal is elicited lower than the cricoid cartilage, it suggests downward displacement of the tube.
- If artifact EMG signal is elicited upper than the middle of the thyroid cartilage, it suggests upward displacement.
The corrective maneuver for malpositioned ETT can be done by the surgeon performing the vagal stimulation while the anesthesiologist readjusts the tube (3). Vagal stimulation should return the robust waveform when correct ETT placement has been established. We routinely use laryngo fiberscope examination to ensure the presence of laryngeal twitch and to adjust electrodes position, when false LOS is suspected (4).
Misuse of NMBAs
Repeated administration of any NMBA intraoperatively results in LOS. Therefore, the surgeon should take a few seconds to re-confirm this issue with the anesthetist when LOS occurs. If a NABA has been inadvertently administered, some waiting time (i.e., 20 to 30 minutes) may be needed to wear off the effect, or reversal agents may be given to allow for resumption of normal muscle twitch activity.
Algorithm of intraoperative LOS
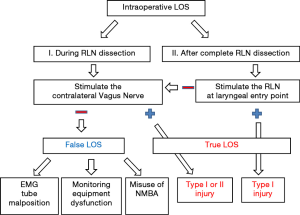
In the scenario of intraoperative LOS, an efficient algorithm (Figure 2) can be followed for determining the causes of LOS:
LOS during RLN dissection
In the scenario of intraoperative LOS and laryngeal entry point of RLN has not yet been exposed, the authors suggest the first step is to perform the contralateral vagus nerve stimulation.
- Negative contralateral vagus nerve stimulation means the monitor is not functionally working, and surgeons should consider this is a false LOS episode and further exclude the common reasons caused by monitoring equipment dysfunction, EMG tube malposition, and misuse of NMBAs.
- Positive contralateral vagus nerve stimulation means the monitor is functionally working, and surgeons should consider this is a true LOS episode.
LOS after complete RLN dissection
If the intraoperative LOS occurs near the end or after complete RLN dissection and the laryngeal entry point has been exposed, the authors suggest the first step is to stimulate the RLN at laryngeal entry point.
- If there is a positive EMG signal at laryngeal entry point but negative signal at proximal point of exposed RLN or vagus nerve, the situation indicates a type I nerve injury. Surgeons can detect the injured point by testing the RLN from laryngeal entry point to proximal area.
- If negative ipsilateral EMG signal at laryngeal entry point but there is positive contralateral vagus nerve stimulation, this excludes monitor dysfunction and suggests a type II (Global) injury lesion.
Although the assessment of laryngeal twitch response by the surgeon is recommended as one of the first step for intraoperative LOS evaluation in the current guideline statement (3), the twitch response may vary among patients (race, sex and age) and the interpretation may vary among surgeons (training, and experience). Since the surgeons can easily perform the contralateral vagus nerve stimulation or RLN stimulation at laryngeal entry point to differentiate the false and true LOS (Figure 2), the authors suggest these procedures may be a more practical and objective initial steps than laryngeal twitch assessment when facing the intraoperative LOS.
Management of the true LOS
Map lesion and elucidate the causes of nerve injury
During thyroid operations, especially during the phase of lateral thyroid and RLN dissection, the RLN can be injured by the surgical maneuvers of transection, traction, clamping, mechanical trauma, or electro-cauterization. In the setting of true LOS, the surgeon should make an effort to identify the injured segment of nerve by serially testing the entire segment of exposed RLN from distal to proximal to see if a neurapraxic segment of LOS can be identified. The identification of such a segment then can allow the surgeons to elucidate the surgical pitfalls that caused the nerve injury and improve their surgical techniques (4,5).
Recheck the EMG at the end of operation
Three possible outcomes of intraoperative true LOS can be observed at the end of operation, including: (I) persistent LOS (no recovery); (II) partial signal recovery (Figure 3); and (III) complete signal recovery, and the outcome of cord mobility, can be either normal, weakened or fixed (5).

Previously, the capability of intraoperative recovery of LOS after RLN injury was under debate, but our recent experimental study has confirmed the phenomenon of intraoperative recovery of severely weakened or lost EMG signals after acute RLN traction, and we found that the degree of recovery is related to the extent of the injury (13). Chiang et al. (5) reported 13 nerves with LOS caused by traction or constricting injuries; the EMG signal regained complete recovery in 3 nerves, partial recovery in 3 and no recovery in 7. The outcome of cord mobility was normal in all nerves with complete recovery and 2 nerves with partial recovery, weak in the remaining nerve with partial recovery, and fixed mobility in all nerves without signal recovery. Sitges-Serra et al. (14) also reported that 15 RLNs had re-entry signal among 16 nerves with intraoperative LOS (over 90%) and only 3 nerves developed temporary vocal palsy. Therefore, intraoperative LOS episode does not always indicate vocal cord paralysis after operation. Some LOS represent transient neuropraxia of short duration and the nerve may regain the signal before the end of operation (13). For preventing unnecessary 2nd operation, 20 minutes of waiting is necessary before making a decision to perform a staged thyroidectomy (14).
Consider optimal contralateral surgery timing
One of the worst complications in thyroid surgery is bilateral RLN paralysis, which can lead to transient or definitive tracheotomy. Sadowski et al. (15) reported the systematic use of IONM and the change in operative strategy will lead to an almost 0% rate of bilateral RLN palsy, at least in benign thyroid conditions. True LOS with no or only a subtle recovery at the end of operation indicates a high risk of at least a postoperative temporary vocal palsy, and therefore surgeon must consider whether a two-stage thyroidectomy is needed in patients with planned bilateral thyroid operation, even in cases of malignancies, to avoid the disaster of bilateral vocal cord palsy.
Follow-up of the post-operative laryngeal examination
Symptomatic assessment of vocal cord paralysis is notoriously inaccurate, as noted previously (16-19). Recognition of preoperative vocal cord paralysis is essential if the surgeon is to reduce the risk of contralateral injury and bilateral cord paralysis (16). Therefore, during standardized IONM, all patients need to have preoperative and postoperative laryngeal examination, best assisted with video-recording, if the true rate of RLN injury is to be appreciated (4). This is especially important for patients experiencing intraoperative true LOS, where more regular postoperative laryngeal examination should be arranged to record and access the function status of vocal cord. When vocal dysfunction is identified, follow-up occurs every 2 weeks initially and every 4 weeks thereafter until recovery is achieved. Generally, dysfunction is considered permanent if it persists 6 months postoperatively.
Surgeons need to realize that false negative result of an intraoperative true LOS can happen if it is very transient and the nerve recovers before laryngeal examination is performed. Dionigi et al. (20) reported that the rate of RLN palsy is influenced by the “timing” of the postoperative laryngoscopy. They also suggest the proper timing of laryngeal inspection is at second day post-op (T2) and the reason of the slightly superior sensitivity of T2 compared to T1 (day 0 in the recovery room) may be associated to patient’s poor compliance, self-adherence, and degree to which he/she correctly follows medical advice during the laryngeal examination early after extubation (T1).
Future perspective
In addition to 100 µV, which is currently used as the threshold value of LOS in most studies (3-5,7,14,21-24), other threshold values for prediction of nerve function impairment have been reported recently [i.e., 200 (25) or 280 µV (26)]. Genther et al. (25) concluded that laryngeal IONM can predict a favorable outcome of laryngeal mobility in cases in which the response is at least 200 µV. Under this value, the risk of immediate postoperative vocal palsy is about 72%. Another study by Pavier et al. (26) reported that the threshold value to assure postoperative laryngeal mobility is 280 µV; the risk of palsy under this value is about 50%. Therefore, some surgeons might choose to use a higher threshold value for LOS (i.e., from 200 to 300 µV). A higher threshold value for LOS might be helpful for early detection of adverse EMG change and for decreasing the artifact during dissection close to the trachea (Chiang and Wu, unpublished data).
In fact, EMG amplitudes during IONM may vary significantly within a patient and among patients. In patients with relatively higher (for example, amplitude >1,500 µV) or lower baseline signals (for example, amplitude <400 µV) than the reported normative data (mean amplitudes of initial vagus nerve or RLN stimulation around 500 to 1,200 µV) (8-10,24,27-30), the false positive or negative rates for prediction of laryngeal mobility outcome would be expected to be high when using the absolute threshold values (100, 200, or 280 µV) for LOS.
In the setting of continuous vagal IONM, surgeons can utilize the monitor software to set lower limit threshold by percentage (%) of amplitude reduction (50% for instance) to alarm the adverse EMG changes, so that they can correct certain maneuvers immediately to prevent irreversible nerve injury (13,21). Therefore, additional work in the future may also consider to use these relative threshold values (i.e., % of amplitude reduction from initial signal) as one of the criteria for the definition of LOS (80% or 90% amplitude reduction for instance) and compare their validity in the prediction of postoperative vocal cord function (Chiang and Wu, unpublished data).
Conclusions
The updated causes, algorithm, and management of LOS during RLN neuromonitoring are reviewed and summarized in this article. Because the threshold criteria of LOS has yet to be clearly and accurately defined, additional studies are needed to compare the validity of using different threshold values (i.e., 100 vs. 200-300 µV vs. % of amplitude reduction) as LOS definition for better prediction of vocal mobility outcome.
Acknowledgements
Funding: This study was supported by grants from the National Science Council Taiwan (MOST 103-2314-B-037-037-MY2), and Kaohsiung Medical University Hospital Taiwan (Kmuh101-1R35, 102-2R33).
Disclosure: The authors declare no conflict of interest.
References
- Chiang FY, Wang LF, Huang YF, et al. Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery 2005;137:342-7. [PubMed]
- Randolph GW. eds. Surgery of the Thyroid and Parathyroid Glands. 2nd ed. Philadelphia, PA: Saunders, 2013.
- Randolph GW, Dralle H; International Intraoperative Monitoring Study Group, Abdullah H, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121 Suppl 1:S1-16. [PubMed]
- Chiang FY, Lee KW, Chen HC, et al. Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg 2010;34:223-9. [PubMed]
- Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery 2008;143:743-9. [PubMed]
- Wu CW, Lu IC, Randolph GW, et al. Investigation of optimal intensity and safety of electrical nerve stimulation during intraoperative neuromonitoring of the recurrent laryngeal nerve: a prospective porcine model. Head Neck 2010;32:1295-301. [PubMed]
- Melin M, Schwarz K, Lammers BJ, et al. IONM-guided goiter surgery leading to two-stage thyroidectomy--indication and results. Langenbecks Arch Surg 2013;398:411-8. [PubMed]
- Dionigi G, Chiang FY, Rausei S, et al. Surgical anatomy and neurophysiology of the vagus nerve (VN) for standardised intraoperative neuromonitoring (IONM) of the inferior laryngeal nerve (ILN) during thyroidectomy. Langenbecks Arch Surg 2010;395:893-9. [PubMed]
- Dionigi G, Alesina PF, Barczynski M, et al. Recurrent laryngeal nerve injury in video-assisted thyroidectomy: lessons learned from neuromonitoring. Surg Endosc 2012;26:2601-8. [PubMed]
- Wu CW, Dionigi G, Chen HC, et al. Vagal nerve stimulation without dissecting the carotid sheath during intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery. Head Neck 2013;35:1443-7. [PubMed]
- Lu IC, Chu KS, Tsai CJ, et al. Optimal depth of NIM EMG endotracheal tube for intraoperative neuromonitoring of the recurrent laryngeal nerve during thyroidectomy. World J Surg 2008;32:1935-9. [PubMed]
- Tsai CJ, Tseng KY, Wang FY, et al. Electromyographic endotracheal tube placement during thyroid surgery in neuromonitoring of recurrent laryngeal nerve. Kaohsiung J Med Sci 2011;27:96-101. [PubMed]
- Wu CW, Dionigi G, Sun H, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: a porcine model. Surgery 2014;155:329-39. [PubMed]
- Sitges-Serra A, Fontané J, Dueñas JP, et al. Prospective study on loss of signal on the first side during neuromonitoring of the recurrent laryngeal nerve in total thyroidectomy. Br J Surg 2013;100:662-6. [PubMed]
- Sadowski SM, Soardo P, Leuchter I, et al. Systematic use of recurrent laryngeal nerve neuromonitoring changes the operative strategy in planned bilateral thyroidectomy. Thyroid 2013;23:329-33. [PubMed]
- Randolph GW, Kamani D. The importance of preoperative laryngoscopy in patients undergoing thyroidectomy: voice, vocal cord function, and the preoperative detection of invasive thyroid malignancy. Surgery 2006;139:357-62. [PubMed]
- Steurer M, Passler C, Denk DM, et al. Advantages of recurrent laryngeal nerve identification in thyroidectomy and parathyroidectomy and the importance of preoperative and postoperative laryngoscopic examination in more than 1000 nerves at risk. Laryngoscope 2002;112:124-33. [PubMed]
- Rueger RG. Benign disease of the thyroid gland and vocal cord paralysis. Laryngoscope 1974;84:897-907. [PubMed]
- Huppler EG, Schmidt HW, Devine KD, et al. Ultimate outcome of patients with vocal-cord paralysis of undetermined cause. Am Rev Tuberc 1956;73:52-60. [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Postoperative laryngoscopy in thyroid surgery: proper timing to detect recurrent laryngeal nerve injury. Langenbecks Arch Surg 2010;395:327-31. [PubMed]
- Schneider R, Randolph GW, Sekulla C, et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013;35:1591-8. [PubMed]
- Jonas J. Continuous vagal nerve stimulation for recurrent laryngeal nerve protection in thyroid surgery. Eur Surg Res 2010;44:185-91. [PubMed]
- Cernea CR, Brandão LG, Hojaij FC, et al. Negative and positive predictive values of nerve monitoring in thyroidectomy. Head Neck 2012;34:175-9. [PubMed]
- Caragacianu D, Kamani D, Randolph GW. Intraoperative monitoring: normative range associated with normal postoperative glottic function. Laryngoscope 2013;123:3026-31. [PubMed]
- Genther DJ, Kandil EH, Noureldine SI, et al. Correlation of final evoked potential amplitudes on intraoperative electromyography of the recurrent laryngeal nerve with immediate postoperative vocal fold function after thyroid and parathyroid surgery. JAMA Otolaryngol Head Neck Surg 2014;140:124-8. [PubMed]
- Pavier Y, Saroul N, Pereira B, et al. Acute prediction of laryngeal outcome during thyroid surgery by electromyographic laryngeal monitoring. Head Neck 2014. [Epub ahead of print]. [PubMed]
- Lorenz K, Sekulla C, Schelle J, et al. What are normal quantitative parameters of intraoperative neuromonitoring (IONM) in thyroid surgery? Langenbecks Arch Surg 2010;395:901-9. [PubMed]
- Potenza AS, Phelan EA, Cernea CR, et al. Normative intra-operative electrophysiologic waveform analysis of superior laryngeal nerve external branch and recurrent laryngeal nerve in patients undergoing thyroid surgery. World J Surg 2013;37:2336-42. [PubMed]
- Chiang FY, Lu IC, Tsai CJ, et al. Does extensive dissection of recurrent laryngeal nerve during thyroid operation increase the risk of nerve injury? Evidence from the application of intraoperative neuromonitoring. Am J Otolaryngol 2011;32:499-503. [PubMed]
- Chu KS, Wu SH, Lu IC, et al. Feasibility of intraoperative neuromonitoring during thyroid surgery after administration of nondepolarizing neuromuscular blocking agents. World J Surg 2009;33:1408-13. [PubMed]


