
Updates in the advances of sporadic medullary thyroid carcinoma: from the molecules to the clinic
Introduction
Medullary thyroid carcinoma (MTC) arises from the parafollicular cells (C cells), and accounts for less than 5% of thyroid malignancies (1,2). As a neuroendocrine tumor, MTC secretes calcitonin, which leads to the elevation of serum calcitonin. Therefore, serum calcitonin becomes a vital marker to monitor the occurrence or recurrence of MTC (3). Histologically, the frequent amyloid deposits and occasional psammoma bodies in the stroma differentiate MTC from other extrathyroid-originated neuroendocrine tumors. Unlike other neuroendocrine tumors, about a quarter of MTC appears as a predominant part of hereditary multiple endocrine neoplasia type 2 (MEN2) syndromes. With the involvement of other synchronous and metachronous lesions (including pheochromocytoma, hyperparathyroidism, and mucosal neuroma), MEN2 is divided into three subtypes: MEN2A, MEN2B, and familial MTC (4). Most patients with MEN2A have germline mutations in the Rearranged during Transfection (RET) C634, whereas patients with MEN2B and FMTC have germline mutations in RET M918T (5,6). Somatic RET mutation is identified in a considerable proportion of sporadic MTCs, and RET M918T is the most frequent genetic change (7-19). RAS point mutations are usually mutually exclusive with RET mutations, and occur in 0–81% of RET-negative MTCs (12,15,17,20-25). Several independent studies have shown that RET and RAS mutations have prognostic significance (7,11,17,25-29). Hence, the prevalence of RET and RAS mutations in different populations might partly explain the difference in prognosis among different populations with sporadic MTC. Many genetic changes are found in the RET and RAS-negative cases, although their molecular mechanism and clinic significance are yet to be determined.
Immune checkpoint inhibitors targeting PD-L1/PD-1 are regarded as one of the most significant medical breakthroughs in the 21st century (30-32). Previous studies have revealed poor tumor-infiltrating lymphocytes, low expression of PD-L1 and low mutational burden in MTCs (24,33,34), which disfavor the application of immunotherapy. However, several reports from China have proved that PD-L1 expression is related to advanced disease. The positive correlation between PD-L1 expression and advanced disease may bring the dawn of immunotherapy in advanced MTC, and relevant clinical trials are expected (35,36). The American FDA has approved two multikinase inhibitor (MKI) drugs, vandetanib and cabozantinib, in the treatment of advanced MTC. A more specific RET-targeting tyrosine kinase inhibitor (TKI) is currently underdeveloped. The combination of TKI therapy and immunotherapy might represent a novel therapeutic option in the treatment of advanced MTC (1).
This article focuses on the advances in genetic alterations and their prognostic impact in sporadic MTC among different populations and discusses the potential role of immunotherapy targeting PD-L1/PD-1. Furthermore, the current multikinase inhibitor target therapy for sporadic MTC is summarized. Updates in advance of the role of calcitonin/procalcitonin/calcitonin-related polypeptide alpha (CALCA) gene transcripts in diagnosing and handling MTC are also mentioned.
RET mutations
The RET gene was firstly described in 1985, as a transforming gene encoding a receptor tyrosine kinase (37,38). RET is localized on 10q11.2, and consists of 21 exons (38,39). RET is a single-pass transmembrane protein, which contains the following functional domains: an extracellular domain with four repeated cadherin-like regions, a cysteine-rich region, a transmembrane domain, a broad intracellular tyrosine kinase domain, and a carboxy-terminal domain (allowing for three isoforms, RET 9, 43, and 51) (Figure 1) (40-42). The intracellular domain encompasses two tyrosine kinase subdomains (TKS1 and TKS2) that are involved in the activation of many intracellular signal transduction pathways (40-42).
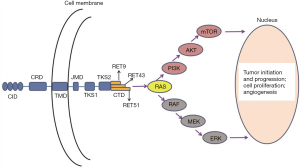
As a receptor tyrosine kinase, RET plays a crucial role in cell signal transduction, and the germline mutation of RET leads to the destruction of cell proliferation and differentiation in tissues derived from neural crest cells, including parafollicular thyroid cells, parathyroid gland, adrenal medulla, and intestinal autonomic nerve plexus (5,40,43). The molecular mechanism of somatic RET mutation is supposed to be like germline mutation (44). RET protein performs its pivotal role in regulating cellular transformation, survival, differentiation, proliferation, and migration. The transformation is done through the binding of its ligand GFRa complex, triggering its homodimerization, phosphorylation of tyrosine residues, and initiation of several intracellular signaling cascades, including MAPK and PI3K pathways (Figure 1) (2,41,42,45,46).
Sporadic MTCs have various somatic gene point mutations and deletions. The prevalence of somatic RET mutations in sporadic MTCs varies from 19.4–88.9% among different populations (4,7-19,27). The lowest mutation rate of somatic RET is from a Chinese population, while the highest from the US population. There is a general agreement that RET T918M, a point mutation in the methionine residue in exon 16 corresponding to the intracellular tyrosine kinase receptor domain, is the most frequent somatic RET mutation, the germline mutation of which was originally known as the activating mutation in MEN 2B. Other mutations in the RET gene in sporadic MTC have been recognized at codons 609, 611, 618, 620, 630, 631, 632, 634, 636, 639, 641, 748, 766, 768, 876, 883, 884, 901, 908, 919, 922, and 930 at exons 10, 11, 13, 14, 15 and 16 (4,8,15,40,47-50).
There are many pieces of evidence that patients with sporadic MTC harboring RET mutations have a poorer prognosis than those harboring non-RET mutations (7,11,17,25-28,51). A statistically significant correlation was reached between RET mutations and the advanced tumor stage, higher T category, and lymph nodes or distant metastases and a worse patient outcome (17). In a recent meta-analysis involving 23 studies with 964 patients with MTC, RET mutation was determined to be associated with an elevated risk for lymph node metastasis, distant metastasis, advanced tumor stage, tumor recurrence, and patient mortality (28). Furthermore, tumors with somatic codon 918 mutations appear more aggressive than tumors with other RET mutations (17). Interestingly, RET mutations in sporadic MTCs may not necessarily lead to tumorigenesis but are essential for disease progression. This behavior is supported by the fact that the incidence of RET mutations in sporadic small MTC (smaller than 1 cm: microMTC) is lower than in larger tumors, and that RET mutations often exhibit mutational heterogeneity even in the advanced disease when present (38,52). Romei et al. hypothesized that either other oncogenes are responsible for most microMTCs, thereby identifying a tumor subpopulation or that RET mutation might or might not occur later during tumor progression (52).
Other somatic changes of RET, including copy number alteration and retrocopy, codon deletions, and rearrangements, were likely to be involved in the pathogenesis and progression of MTCs (17,47,53-55). Bim et al. identified copy number alteration and retrocopy in the RET gene; determined a recurrent novel point mutation (G548V) exclusively in the somatic retrocopy of RET in both sporadic and hereditary MTCs and MTC cell lines, and identified retrocopies produced in somatic cells might play a role in the pathogenesis and progression of MTCs (53). A rare RET fusion, MYH13 exon 35 with RET exon 12, was reported in a patient who died of aggressive sporadic MTC with the survival of fewer than ten months after diagnosis, which might be a novel driver genetic change, and a RET fusion also provides a possible target for the treatment of RET TKI (54).
RAS mutations
The RAS (P21) protein is located on the inside of the cell membrane and plays a vital role in the transduction of cell growth and differentiation signals (56). It belongs to the guanosine triphosphate (GTP) binding protein (a coupling factor of cell information transmission), which regulates information transduction through the transformation between GTP and guanosine diphosphate (GDP) (56,57). There are three members of the RAS gene family: HRAS, KRAS, and NRAS (58). The various RAS genes are found on different chromosomes but have similar structures, all composed of four exons. RAS takes part in both MAPK and PI3K signal pathways. RAS point mutations in MTC occur in HRAS and KRAS within hot spots in exons 2, 3, and 4, and rarely involve NRAS. They are usually mutually exclusive with RET mutations and are present in approximately 0–81% of RET wild-type sporadic MTCs (15,17,20-25), suggesting that RET and RAS somatic mutations are likely mutually exclusive in MTC tumorigenesis and development. Patients harboring RAS mutations (RET negative) showed a better prognosis than those harboring RET mutations or presenting no mutations (17,29).
However, the outcome of patients with a somatic RET mutation was significantly worse than both RAS positive/RET negative and RAS negative/RET negative cases (13,17). RAS mutations were reported to be significantly associated with higher intensity of p-S6 expression, suggesting that the mTOR pathway was activated in such MTCs (25). These findings suggest RAS mutations could play a role in tumor development, which leads to a less aggressive tumor phenotype, whereas RET mutations could be responsible for a more aggressive phenotype associated with a worse prognosis (22,27).
Other genetic alterations
Other somatic mutations were found in a subset of patients with MTC, especially patients without RET and RAS mutations. Ciampi et al. identified somatic mutations in the genes of MET, TP53, TSH receptor, EIF1AX, CHK2, PPM1D (29). In a study of MTC in Taiwan’s population, they identified ten novels MTC somatic mutations: BICD2, DLG1, FSD2, IL17RD, KLHL25, PAPPA2, PRDM2, PSEN1, SCRN1, and TTC1, using whole-exome sequencing (33). They further analyzed 1,152 MTCs from COSMIC data and found that most of the variants were involved in pathways of PI3k-Akt, ErbB, MAPK, mTOR, and VEGF signaling pathways, and some were included in the pathogenesis of thyroid cancer, central carbon metabolism, and microRNAs in cancer (33). FAT1 and FAT4, two members of the FAT gene family, both located at chromosome 4q, were identified as the two most commonly mutated genes in addition to RET in a small cohort of 18 cases of sporadic MTCs from China by Qu et al. They further showed that FAT1 and FAT4 knockdowns could promote MTC cell proliferation, using TT and MZ-CRC-1 cell lines (15). It is a novel finding that, on the gene expression profile, MTCs could be clustered into two molecular subtypes: the mesenchymal-like subtype, characterized by the epithelial-mesenchymal transition, and the proliferative-like subtype, associated with enrichment of cell cycle pathways. Most events of structural recurrence occur in the latter (15).
Copy-number losses in chromosome 4q and 1p cause frequent somatic changes for patients with sporadic MTC (15). A study in MD Anderson Cancer Center reported that 19% of patients with sporadic MTC had an aberrant loss of the cyclin-dependent-kinase inhibitors 2C (CDKN2C), found in the 1p32 chromosomal region. The aberrant CDKN2C loss was associated with distant metastasis at presentation and an unfavorable overall survival (OS). These findings were explained by showing that aberrant loss of CDKNs could lead to unrestrained phosphorylation of retinoblastoma protein and unregulated progression through the S phase of the cell cycle, thus resulting in the development of cancers. They also demonstrated the clinical impact of aberrant CDKN2C loss was enhanced by concomitant RET M918 mutation (59). Anaplastic lymphoma kinase (ALK) translocation, a frequent genetic alteration in anaplastic large cell lymphoma and lung adenocarcinoma, was identified in two cases by Ji et al. from screening 98 cases of MTC, with the partner genes of EML4 and GFPT1, respectively (60). This finding may show that a rare subset of patients with MTC with ALK fusion might receive help from ALK target therapy. No NTRK translocation has been reported in MTC, although NTRK mutation has been reported in MTC (61).
Tumor microenvironment and immunotherapy
In the past decade, people have achieved gratifying results through using immunotherapy in treating many solid cancers, which was an essential milestone in the history of medical development (62). The successful findings in tumor immunotherapy are due to the discovery of immune checkpoint molecules, which are especially important in the regulation of tumor microenvironments. The tumor microenvironment is the internal environment in which tumor cells are produced and live. It consists of not only tumor cells but also fibroblasts, immune and inflammatory cells, and other cells in close contact with tumor cells, as well as adjacent cell stroma, microvessels, and infiltrated biological molecules (42,63-65). Malignant cells actively take part in the reconstruction of a pre-existing matrix, creating a new microenvironment that can be characterized by inflammation or desmoplastic characteristics. Tumor microenvironment and cancer cells interact and influence each other. They are thereby promoting tumor progression and metastasis (42,63-65). It has been proposed to classify tumor microenvironments into four groups on their status of PD-L1 and tumor-infiltrating lymphocytes to streamline immunotherapy (31).
MTCs often manifest as few tumor-infiltrating immune cells with low expression of PD-L1 both in the tumor and in the stroma cells (34,35), and a low mutational rate (24). However, there is also evidence showing a positive immune reactivity of MTC. In a large cohort of 201 consecutive Chinese patients with MTC, Shi et al. demonstrated PD-L1 positivity was associated with aggressive clinicopathologic features (a larger tumor size, lymph node metastases, and advanced TNM staging) and is independently predictive of structural recurrence, and biochemical recurrence/persistent disease (36). Furthermore, they revealed a higher rate of PD-L1 expression in patients with incurable recurrence (36). Bi et al. have reported similar findings (35). They detected the expression of PD-L1 and PD-1 in 87 cases of MTC and found PD-L1 positivity was significantly correlated with distant metastases at the surgery. That co-expression of PD-1 and PD-L1 in MTC was correlated with advanced pathologic TNM stage III/IV and distant metastases at the surgery. The positive correlation between PD-1/PD-L1 and prognosis suggests that immunotherapy targeting PD-L1/PD-1 might be effective in treating advanced MTC. Several ongoing trials of immunotherapy in treating advanced MTC are under study, but no definite result has been reported. Although the FDA has approved several anti-angiogenic multikinase inhibitors (aaMKIs) in treating advanced MTC (discussed below), novel methods are still required to treat patients who fail to respond to aaMKI. Immunotherapy might be a choice in the future (66).
Targeting therapy in MTC
By far, surgery is still to be the only therapeutic method in treating most MTCs. Although radioactive iodine and TSH-suppressive therapies can treat differentiated thyroid carcinoma, they have no role in the management of MTC since the neuroendocrine-derived tumor cells do not concentrate iodine or respond to TSH in the same manner that follicular cells do. With the development of targeted therapy in treating advanced or unresectable MTC two aaMKIs, vandetanib, and the American FDA approved cabozantinib for the treatment of advanced MTC in 2011 and 2012, respectively. In the phase III trials of both drugs, patients with metastatic MTC had a significantly longer median progression-free survival (PFS) in the treatment group than in the placebo group (67-69). Both of the drugs did not show their efficacy in improving the OS of patients (67-70). Nevertheless, both studies met their pre-specified primary endpoints for improving PFS and were thus approved by the FDA as the first effective drugs for the treatment of advanced MTC.
Further, evidence showed that cabozantinib provided the most significant clinical benefit to patients with RET M918T or RAS mutation (71). The most common adverse drug reactions for vandetanib and cabozantinib were diarrhea, rashes, nausea, hypertension, and headaches (67,69,72,73). It is worth noting QT prolongation represented a rare but critical adverse event in the therapy of vandetanib, which potentially evolved into torsade de pointes and sudden death (67,73). Hence, the drug can only be prescribed by qualified physicians and should be used under close surveillance. Despite the advances in the management of metastatic MTC, aaMKIs show significant off-target toxicity and limited efficacy (67,69). LOXO-292 and BLU-667, a new generation of small-molecule TKIs, are highly selective RET inhibitors, and both of them showed better therapeutic efficacy than approved MKIs in the preliminary studies (74-77). At present, clinical trials of both are currently under evaluation (76).
The role of calcitonin/procalcitonin/CALCA gene transcripts in diagnosing and handling MTC
Calcitonin is a hormone that regulates the level of serum calcium, secreted by the C cells of the thyroid and metabolized by the kidneys (78). Calcitonin is regarded as a sensitive marker for diagnosing MTC, and is used for postoperative monitoring of MTC patients (3,79-81). However, the role of routine calcitonin screening in patients with thyroid nodules is still questionable, since the incidence of MTC is low and other non-neoplastic and neoplastic conditions may cause the increase of serum calcitonin (79,82,83). Calcitonin measurement in aspiration needle washout of thyroid nodules and neck lesions is reported to have a higher sensitivity than traditional cytology in diagnosing MTC. It may represent an ancillary tool utilized in patients with high serum calcitonin to avoid false-negative MTC by cytology (83,84). A large-scale retrospective analysis shows that postoperative biochemical remission of serum calcitonin is significantly correlated with a decrease of the 5-year recurrence rate, but not with the improved 5-year survival rate (81).
Procalcitonin is the precursor of calcitonin. It is widely used as an indicator of severe infection, sepsis, and multiple organ failure (85-87). In recent years, a growing body of research has shown that procalcitonin presents an equivalent or even superior alternative of calcitonin in the management of MTC (88-92). Camacho et al. show that the expression of CALCA mRNA from the peripheral blood presents higher sensitivity, specificity, together with higher positive predictive value, and negative predictive value than did serum calcitonin (93). Thus, the detection of CALCA mRNA expression could serve as an alternative to the calcitonin-stimulation test since the former is more convenient to perform (93).
Conclusions
Advanced MTC is still one of the most challenging malignancies for clinicians. With the increasing development of molecular detection techniques, many genetic alterations have been discovered, while RET and RAS point mutations are still the most frequent and most significant changes. There is growing evidence that genetic changes might be used to predict the clinical outcome of patients with sporadic MTC, on different RET point mutations and RAS mutations. The in-depth understanding of molecular mechanisms and immune microenvironments of sporadic MTC has prompted the development of targeted therapy and immunotherapy. It is promising that the combination of TKI-targeted therapy and immune checkpoint inhibitors might be a novel therapeutic approach for treating patients with advanced MTC according to the individual tumor mutation profile and tumor microenvironment.
Acknowledgments
Funding: This article was supported by the Beijing Municipal Administration of Hospitals Incubating Program, Code: PX2018042.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kennichi Kakudo) for the series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-2019-catp-21). The series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” was commissioned by the editorial office without any funding or sponsorship. KK served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Gland Surgery from Sep 2018 to Aug 2020. YB reports grants from Beijing Municipal Administration of Hospitals Incubating Program, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cakir M, Grossman AB. Medullary thyroid cancer: molecular biology and novel molecular therapies. Neuroendocrinology 2009;90:323-48. [Crossref] [PubMed]
- Giunti S, Antonelli A, Amorosi A, et al. Cellular signaling pathway alterations and potential targeted therapies for medullary thyroid carcinoma. Int J Endocrinol 2013;2013:803171. [Crossref] [PubMed]
- Grozinsky-Glasberg S, Benbassat CA, Tsvetov G, et al. Medullary thyroid cancer: a retrospective analysis of a cohort treated at a single tertiary care center between 1970 and 2005. Thyroid 2007;17:549-56. [Crossref] [PubMed]
- Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994;367:375-6. [Crossref] [PubMed]
- Eng C. RET proto-oncogene in the development of human cancer. J Clin Oncol 1999;17:380-93. [Crossref] [PubMed]
- Zhou Y, Zhao Y, Cui B, et al. RET proto-oncogene mutations are restricted to codons 634 and 918 in mainland Chinese families with MEN2A and MEN2B. Clin Endocrinol (Oxf) 2007;67:570-6. [Crossref] [PubMed]
- Dvorakova S, Vaclavikova E, Sykorova V, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinomas. Mol Cell Endocrinol 2008;284:21-7. [Crossref] [PubMed]
- Marsh DJ, Learoyd DL, Andrew SD, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 1996;44:249-57. [Crossref] [PubMed]
- Kebebew E, Ituarte PH, Siperstein AE, et al. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88:1139-48. [Crossref] [PubMed]
- Romei C, Cosci B, Renzini G, et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC). Clin Endocrinol (Oxf) 2011;74:241-7. [Crossref] [PubMed]
- Mian C, Pennelli G, Barollo S, et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: a useful tool for patient risk stratification. Eur J Endocrinol 2011;164:971-6. [Crossref] [PubMed]
- Nikiforova MN, Wald AI, Roy S, et al. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab 2013;98:E1852-60. [Crossref] [PubMed]
- Simbolo M, Mian C, Barollo S, et al. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch 2014;465:73-8. [Crossref] [PubMed]
- Sharma BP, Saranath D. RET gene mutations and polymorphisms in medullary thyroid carcinomas in Indian patients. J Biosci 2011;36:603-11. [Crossref] [PubMed]
- Qu N, Shi X, Zhao JJ, et al. Genomic and Transcriptomic Characterization of Sporadic Medullary Thyroid Carcinoma. Thyroid 2020;30:1025-36. [Crossref] [PubMed]
- Wei S. Detection of Molecular Alterations in Medullary Thyroid Carcinoma Using Next-Generation Sequencing: an Institutional Experience. Endocr Pathol 2016;27:359-62. [Crossref] [PubMed]
- Ciampi R, Mian C, Fugazzola L, et al. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid 2013;23:50-7. [Crossref] [PubMed]
- Romei C, Casella F, Tacito A, et al. New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations. J Med Genet 2016;53:729-34. [Crossref] [PubMed]
- Hedayati M, Zarif Yeganeh M, Sheikholeslami S, et al. Diversity of mutations in the RET proto-oncogene and its oncogenic mechanism in medullary thyroid cancer. Crit Rev Clin Lab Sci 2016;53:217-27. [Crossref] [PubMed]
- Oczko-Wojciechowska M, Pfeifer A, Rusinek D, et al. The prevalence of somatic RAS mutations in medullary thyroid cancer - a Polish population study. Endokrynol Pol 2015;66:121-5. [Crossref] [PubMed]
- Boichard A, Croux L, Al Ghuzlan A, et al. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab 2012;97:E2031-5. [Crossref] [PubMed]
- Moura MM, Cavaco BM, Pinto AE, et al. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab 2011;96:E863-8. [Crossref] [PubMed]
- Accardo G, Conzo G, Esposito D, et al. Genetics of medullary thyroid cancer: An overview. Int J Surg 2017;41:S2-6. [Crossref] [PubMed]
- Agrawal N, Jiao Y, Sausen M, et al. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab 2013;98:E364-9. [Crossref] [PubMed]
- Lyra J, Vinagre J, Batista R, et al. mTOR activation in medullary thyroid carcinoma with RAS mutation. Eur J Endocrinol 2014;171:633-40. [Crossref] [PubMed]
- Fussey JM, Vaidya B, Kim D, et al. The role of molecular genetics in the clinical management of sporadic medullary thyroid carcinoma: A systematic review. Clin Endocrinol (Oxf) 2019;91:697-707. [Crossref] [PubMed]
- Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 2008;93:682-7. [Crossref] [PubMed]
- Vuong HG, Odate T, Ngo HTT, et al. Clinical significance of RET and RAS mutations in sporadic medullary thyroid carcinoma: a meta-analysis. Endocr Relat Cancer 2018;25:633-41. [Crossref] [PubMed]
- Ciampi R, Romei C, Ramone T, et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 2019;20:324-36. [Crossref] [PubMed]
- Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 2013;19:1021-34. [Crossref] [PubMed]
- Teng MW, Ngiow SF, Ribas A, et al. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res 2015;75:2139-45. [Crossref] [PubMed]
- Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett 2016;370:85-90. [Crossref] [PubMed]
- Chang YS, Chang CC, Huang HY, et al. Detection of Molecular Alterations in Taiwanese Patients with Medullary Thyroid Cancer Using Whole-Exome Sequencing. Endocr Pathol 2018;29:324-31. [Crossref] [PubMed]
- Bongiovanni M, Rebecchini C, Saglietti C, et al. Very low expression of PD-L1 in medullary thyroid carcinoma. Endocr Relat Cancer 2017;24:L35-8. [Crossref] [PubMed]
- Bi Y, Ren X, Bai X, et al. PD-1/PD-L1 expressions in medullary thyroid carcinoma: Clinicopathologic and prognostic analysis of Chinese population. Eur J Surg Oncol 2019;45:353-8. [Crossref] [PubMed]
- Shi X, Yu PC, Lei BW, et al. Association Between Programmed Death-Ligand 1 Expression and Clinicopathological Characteristics, Structural Recurrence, and Biochemical Recurrence/Persistent Disease in Medullary Thyroid Carcinoma. Thyroid 2019;29:1269-78. [Crossref] [PubMed]
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985;42:581-8. [Crossref] [PubMed]
- Eng C, Mulligan LM, Healey CS, et al. Heterogeneous mutation of the RET proto-oncogene in subpopulations of medullary thyroid carcinoma. Cancer Res 1996;56:2167-70. [PubMed]
- Pasini B, Hofstra RM, Yin L, et al. The physical map of the human RET proto-oncogene. Oncogene 1995;11:1737-43. [PubMed]
- de Groot JW, Links TP, Plukker JT, et al. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev 2006;27:535-60. [Crossref] [PubMed]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 2002;3:383-94. [Crossref] [PubMed]
- Castellone MD, Melillo RM. RET-mediated modulation of tumor microenvironment and immune response in multiple endocrine neoplasia type 2 (MEN2). Endocr Relat Cancer 2018;25:T105-19. [Crossref] [PubMed]
- Ponder BA. The phenotypes associated with ret mutations in the multiple endocrine neoplasia type 2 syndrome. Cancer Res 1999;59:1736s-41s; discussion 1742s. [PubMed]
- Ameur N, Lacroix L, Roucan S, Roux V, et al. Aggressive inherited and sporadic medullary thyroid carcinomas display similar oncogenic pathways. Endocr Relat Cancer 2009;16:1261-72. [Crossref] [PubMed]
- Lai AZ, Gujral TS, Mulligan LM. RET signaling in endocrine tumors: delving deeper into molecular mechanisms. Endocr Pathol 2007;18:57-67. [Crossref] [PubMed]
- Boikos SA, Stratakis CA. Molecular mechanisms of medullary thyroid carcinoma: current approaches in diagnosis and treatment. Histol Histopathol 2008;23:109-16. [PubMed]
- Alemi M, Lucas SD, Sällström JF, et al. A complex nine base pair deletion in RET exon 11 common in sporadic medullary thyroid carcinoma. Oncogene 1997;14:2041-5. [Crossref] [PubMed]
- Shirahama S, Ogura K, Takami H, et al. Mutational analysis of the RET proto-oncogene in 71 Japanese patients with medullary thyroid carcinoma. J Hum Genet 1998;43:101-6. [Crossref] [PubMed]
- Kalinin VN, Amosenko FA, Shabanov MA, et al. Three novel mutations in the RET proto-oncogene. J Mol Med 2001;79:609-12. [Crossref] [PubMed]
- Jindrichova S, Kodet R, Krskova L, et al. The newly detected mutations in the RET proto-oncogene in exon 16 as a cause of sporadic medullary thyroid carcinoma. J Mol Med 2003;81:819-23. [Crossref] [PubMed]
- Romei C, Elisei R, Pinchera A, et al. Somatic mutations of the ret protooncogene in sporadic medullary thyroid carcinoma are not restricted to exon 16 and are associated with tumor recurrence. J Clin Endocrinol Metab 1996;81:1619-22. [PubMed]
- Romei C, Ugolini C, Cosci B, et al. Low prevalence of the somatic M918T RET mutation in micro-medullary thyroid cancer. Thyroid 2012;22:476-81. [Crossref] [PubMed]
- Bim LV, Navarro FCP, Valente FOF, et al. Retroposed copies of RET gene: a somatically acquired event in medullary thyroid carcinoma. BMC Med Genomics 2019;12:104. [Crossref] [PubMed]
- Grubbs EG, Ng PK, Bui J, et al. RET fusion as a novel driver of medullary thyroid carcinoma. J Clin Endocrinol Metab 2015;100:788-93. [Crossref] [PubMed]
- Ciampi R, Romei C, Cosci B, et al. Chromosome 10 and RET gene copy number alterations in hereditary and sporadic Medullary Thyroid Carcinoma. Mol Cell Endocrinol 2012;348:176-82. [Crossref] [PubMed]
- Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst 2001;93:1062-74. [Crossref] [PubMed]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature 1993;366:643-54. [Crossref] [PubMed]
- Barbacid M. ras genes. Annu Rev Biochem 1987;56:779-827. [Crossref] [PubMed]
- Grubbs EG, Williams MD, Scheet P, et al. Role of CDKN2C Copy Number in Sporadic Medullary Thyroid Carcinoma. Thyroid 2016;26:1553-62. [Crossref] [PubMed]
- Ji JH, Oh YL, Hong M, et al. Identification of Driving ALK Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer. PLoS Genet 2015;11:e1005467. [Crossref] [PubMed]
- Gimm O, Greco A, Hoang-Vu C, et al. Mutation analysis reveals novel sequence variants in NTRK1 in sporadic human medullary thyroid carcinoma. J Clin Endocrinol Metab 1999;84:2784-7. [Crossref] [PubMed]
- Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016;13:473-86. [Crossref] [PubMed]
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 2010;18:884-901. [Crossref] [PubMed]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell 2012;21:309-22. [Crossref] [PubMed]
- McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014;16:717-27. [Crossref] [PubMed]
- Hadoux J, Pacini F, Tuttle RM, et al. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol 2016;4:64-71. [Crossref] [PubMed]
- Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134-41. [Crossref] [PubMed]
- Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639-46. [Crossref] [PubMed]
- Krajewska J, Olczyk T, Jarzab B. Cabozantinib for the treatment of progressive metastatic medullary thyroid cancer. Expert Rev Clin Pharmacol 2016;9:69-79. [Crossref] [PubMed]
- Tappenden P, Carroll C, Hamilton J, et al. Cabozantinib and vandetanib for unresectable locally advanced or metastatic medullary thyroid cancer: a systematic review and economic model. Health Technol Assess 2019;23:1-144. [Crossref] [PubMed]
- Sherman SI, Clary DO, Elisei R, et al. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer 2016;122:3856-64. [Crossref] [PubMed]
- Milling RV, Grimm D, Krüger M, et al. Pazopanib, Cabozantinib, and Vandetanib in the Treatment of Progressive Medullary Thyroid Cancer with a Special Focus on the Adverse Effects on Hypertension. Int J Mol Sci 2018;19:3258. [Crossref] [PubMed]
- Hu MI, Elisei R, Dedecjus M, et al. Safety and efficacy of two starting doses of vandetanib in advanced medullary thyroid cancer. Endocr Relat Cancer 2019;26:241-50. [Crossref] [PubMed]
- Subbiah V, Gainor JF, Rahal R, et al. RETPrecision Targeted Therapy with BLU-667 for -Driven Cancers. Cancer Discov 2018;8:836-49. [Crossref] [PubMed]
- Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 2018;29:1869-76. [Crossref] [PubMed]
- Ceolin L, Duval MADS, Benini AF, et al. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer 2019;26:R499-518. [Crossref] [PubMed]
- Zhao Z, Fu T, Gao J, et al. Identifying novel oncogenic RET mutations and characterising their sensitivity to RET-specific inhibitors. J Med Genet 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Felsenfeld AJ, Levine BS. Calcitonin, the forgotten hormone: does it deserve to be forgotten? Clin Kidney J 2015;8:180-7. [Crossref] [PubMed]
- Verbeek HH, de Groot JWB, Sluiter WJ, et al. Calcitonin testing for detection of medullary thyroid cancer in people with thyroid nodules. Cochrane Database Syst Rev 2020;3:CD010159. [Crossref] [PubMed]
- Danila R, Livadariu R, Branisteanu D. Calcitonin revisited in 2020. Acta Endocrinol (Buchar) 2019;15:544-8. [Crossref] [PubMed]
- Jung KY, Kim SM, Yoo WS, et al. Postoperative biochemical remission of serum calcitonin is the best predictive factor for recurrence-free survival of medullary thyroid cancer: a large-scale retrospective analysis over 30 years. Clin Endocrinol (Oxf) 2016;84:587-97. [Crossref] [PubMed]
- Silvestre C, Sampaio Matias J, Proença H, et al. Calcitonin Screening in Nodular Thyroid Disease: Is There a Definitive Answer? Eur Thyroid J 2019;8:79-82. [Crossref] [PubMed]
- Costante G, Meringolo D. Calcitonin as a biomarker of C cell disease: recent achievements and current challenges. Endocrine 2020;67:273-80. [Crossref] [PubMed]
- Trimboli P, Cremonini N, Ceriani L, et al. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: a retrospective multicentre study. Clin Endocrinol (Oxf) 2014;80:135-40. [Crossref] [PubMed]
- Iankova I, Thompson-Leduc P, Kirson NY, et al. Efficacy and Safety of Procalcitonin Guidance in Patients With Suspected or Confirmed Sepsis: A Systematic Review and Meta-Analysis. Crit Care Med 2018;46:691-8. [Crossref] [PubMed]
- Dou YH, Du JK, Liu HL, et al. The role of procalcitonin in the identification of invasive fungal infection-a systemic review and meta-analysis. Diagn Microbiol Infect Dis 2013;76:464-9. [Crossref] [PubMed]
- Honore PM, David C, Attou R, et al. Procalcitonin to allow early detection of sepsis and multiple organ failure in severe multiple trauma: beware of some confounders. Crit Care 2020;24:9. [Crossref] [PubMed]
- Algeciras-Schimnich A, Preissner CM, et al. Procalcitonin: a marker for the diagnosis and follow-up of patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 2009;94:861-8. [Crossref] [PubMed]
- Woliński K, Kaznowski J, Klimowicz A, et al. Diagnostic value of selected biochemical markers in the detection of recurrence of medullary thyroid cancer - comparison of calcitonin, procalcitonin, chromogranin A, and carcinoembryonic antigen. Endokrynol Pol 2017;68:434-7. [Crossref] [PubMed]
- Giovanella L, Imperiali M, Piccardo A, et al. Procalcitonin measurement to screen medullary thyroid carcinoma: A prospective evaluation in a series of 2705 patients with thyroid nodules. Eur J Clin Invest 2018;48:e12934. [Crossref] [PubMed]
- Walter MA, Meier C, Radimerski T, et al. Procalcitonin levels predict clinical course and progression-free survival in patients with medullary thyroid cancer. Cancer 2010;116:31-40. [PubMed]
- Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab 2014;99:2986-94. [Crossref] [PubMed]
- Camacho CP, Lindsey SC, Melo MC, et al. Measurement of calcitonin and calcitonin gene-related peptide mRNA refines the management of patients with medullary thyroid cancer and may replace calcitonin-stimulation tests. Thyroid 2013;23:308-16. [Crossref] [PubMed]


