
The role of multimodal ultrasonic flow imaging in Thyroid Imaging Reporting and Data System (TI-RADS) 4 nodules
Introduction
Thyroid nodules are extremely common. Preoperative assessment of the nature of these nodules provides an important basis for the choice of therapeutic approaches. Ultrasound is used as the modality of choice for nodule detection. Radiologists evaluate the nature of thyroid nodules using the Thyroid Imaging Reporting and Data System (TI-RADS) classification based on the two-dimensional (2D) manifestation of the nodules. The TI-RADS classification proposed by Horvath in 2009 (1), which all thyroid nodules are divided into 6 categories. It is based on the American Society of Radiology Breast Imaging Report and Data System (BI-RADS) (2). However, morphological images alone can be insufficient in accurately diagnosing nodule characteristics. Vessel distribution and flow characteristics within the nodules are widely believed to have a role in defining tumor characteristics (3-5). If tiny blood vessels are detected in a nodule, it indicates that the nodule has a malignant tendency. Therefore, the blood flow signs need to be included in the TI-RADS system evaluation. Microvascular imaging technology has also been applied to the TI-RADS system, and the combination of the two is used to identify malignant nodules. Current methods for depicting blood flow in thyroid nodules include color Doppler imaging (CDFI), contrast-enhanced ultrasound (CEUS), and superb micro-vascular imaging (SMI). CDFI is prone to lose low-frequency and low-velocity flow signal, whereas CEUS can image low flow signals with a diameter of 10–30 µm and a flow velocity of about 1 mm/s. However, the additional contrast agent needed for CEUS is expensive and poses a risk for allergic reaction. SMI was recently introduced as a noninvasive and economical technology for visualizing microflow and can favorably segment tissue signals and minimize motion artefacts. Despite these advantages, there are few reports regarding SMI efficacy in thyroid nodule characterization (6). Here, we observed the characteristics of nodule microvessels using CDFI, CEUS, and SMI, and compared differences between nodule inspection outcomes using single and multiple approaches to illustrate the potential value of SMI in TI-RADS 4 nodules.
We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/gs-20-641).
Methods
Patients
A total of 57 consecutive patients (19 male and 38 female patients; mean age, 45.26±12.30; age range, 24–73 years) comprising 75 thyroid nodules were recruited between April 2016 and January 2018. The inclusion criteria were as follows: (I) confirmation of nodule pathology following surgical resection, and (II) classification of TI-RADS 4 by conventional ultrasound. Among the 75 nodules examined for this study, 55, 18, and 2 nodules were diagnosed as 4a, 4b, and 4c subclass, respectively. Nodules showing circular, coarse, and/or eggshell calcification that could influence SMI imaging were excluded from the study. Written informed consent was obtained from all study subjects. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by Shanghai East Hospital, Tongji University School of Medicine (No. 102).
Instruments and methods
Patients underwent ultrasonic examinations in the supine position with the neck sufficiently exposed. Each patient also was also clinically examined by CDFI, CEUS, and SMI to reveal additional information about localized microflow in the selected nodules. Two-D features of the thyroid nodules were observed, including size, morphology, border, internal echo, aspect ratio, and calcification. The collected 2-D features were then appraised according to guidelines recommended by the American College of Radiology, TI-RADS classification was executed, and nodules having a TI-RADS score of 4 were chosen. Sections with the most abundant flow according to CDFI were selected, and SMI technology was initiated. SMI was applied with a frame frequency of 25–45 fps, a depth of 4–5 cm, an image gain between 50% and 70%, a 2-D dynamic range of 50–65 dB, and a frequency of 7 Hz. CEUS was performed by injecting 1.5 mL of the ultrasound contrast SonoVue into the cubital vein. All examinations were performed by two experienced radiologists using an AplioTM 500 instrument equipped with a 5–14 MHz linear array probe. The first arm of the study involved defining the characteristics of the 2-D presentation in 75 thyroid nodules. In the second arm of the study, CDFI, SMI, and CEUS evaluation of the nodules was conducted. Finally, the results were recorded according to Digital and Imaging Communications in Medicine (DICOM) standards, and final decisions were reached by consensus.
Diagnostic criteria
TI-RADS was used to guide the diagnosis of thyroid nodules in this study (7). Kwak et al. (8) suggested that TI-RADS 4 nodules with solid echogenicity have the following ultrasound signs: hypoechoic/very hypoechoic, blurred border/spicular/irregular shape, microcalcification and aspect ratio >1, and can be divided into three subclasses: 4a, 4b, and 4c, which have one, two, and three or four signs, respectively. The vascular pattern in thyroid nodules can be divided into defined categories according to Kim et al. (5): type I, few (l–2 punctate or short rod signal) or absence of blood flow; type II, ≥3 peripheral vascularity; type III, ≥3 central vascularity; type IV, both type II and III characteristics. The contrast mode of the nodules can be characterized as having no enhancement, hypo-enhancement, iso-enhancement, or hyper-enhancement.
Here, we established a new TI-RADS classification, based on findings by several experienced radiologists and those reported in recent studies (9-12), which adds CDFI, SMI, and CEUS features to the old TI-RADS classification proposed by Kwak et al. In this updated TI-RADS classification, benign tendencies were deemed to present as iso-enhancement, hyper-enhancement or rim enhancement in CEUS and type II/IV flow in SMI, and thus downgraded the classification. In contrast, malignant tendencies would manifest as hypo-enhancement and type III flow, and in turn upgrade the classification. The remaining classifications remain unchanged. Using the updated TI-RADS, when the TI-RADS subclass results obtained by SMI and CEUS were not unanimous, the higher subclass was defined as the final result. Results obtained with the new classification were compared with those using the old TI-RADS classification.
Statistical analysis
Data analyses were conducted using SPSS 19.0 software, with a P value <0.05 considered to indicate statistical significance. Enumeration data between the two groups was checked by Chi-square test. To compare pathological results of the new and old TI-RADS classifications, sensitivity, specificity, and other indicators of CEUS, SMI, and CEUS + SMI were calculated, the receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) was compared by a Z test. Risk-scoring models for different ultrasound methods were built to filter real factors.
Results
Pathology
Among 75 thyroid nodules considered, pathological examination yielded 40 malignant nodules [papillary thyroid carcinoma (PTC), 2 with coexistent Hashimoto’s thyroiditis and a maximum diameter of 0.4–1.5 cm] and 35 benign nodules (maximum diameter 0.6–2.8 cm); 27 were nodular goiters, 4 were thyroid adenomas, and 4 were Hashimoto’s thyroiditis.
SMI and CDFI results
There were significant differences observed between SMI and CDFI outcomes in the inspection of blood flow in benign and malignant nodules (Table 1). SMI was more sensitive in detecting low-velocity flow compared to CDFI (Tables 1-3). Type II and IV flow could be easily seen with SMI in benign nodules compared to CDFI, but the differences were statistically significant only for type II (Table 2 and Figure 1). Meanwhile, SMI could detect type III flow in malignant nodules (Table 3 and Figure 2).
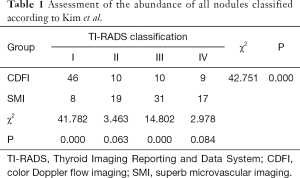
Full table
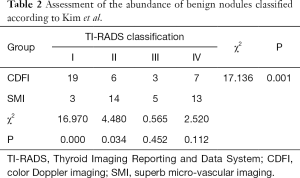
Full table
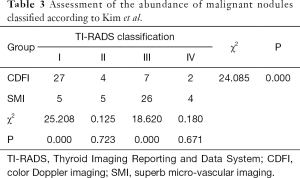
Full table
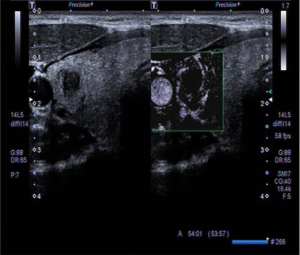
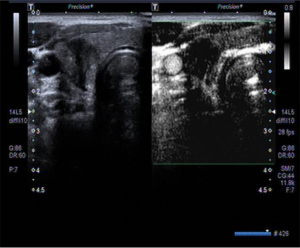
CEUS results
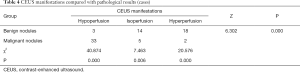
Malignant nodules showed low enhancement by CEUS, whereas benign nodules showed equal or high enhancement. There was a significant difference in perfusion inconsistency between benign and malignant thyroid nodules, and no nodule showed a lack of enhancement (Table 4). Rim type enhancement was included in iso-enhancement and hyper-enhancement. Among the 35 benign nodules, three exhibited rim enhancement, whereas the 40 malignant nodules showed no rim enhancement. The rim enhancement ratio between the two groups showed no significant difference (χ2=1.688, P=0.194).
Full table
New and old TI-RADS classification
After using CDFI, SMI, CEUS, and CEUS+SMI for the new TI-RADS classification, the subclass ratios of downgraded benign nodules were about 1/3, 2/3, 4/5, and 3/5, while the subclass with upgraded ratios of malignant nodules had ratios of approximately 1/4, 1/2, 3/5, and 1/2, respectively (Table 5). Few malignant nodules were present in the downgraded subclass, whereas few benign nodules were present in the upgraded subclass.

Full table
New and old TI-RADS classification AUC and results of diagnostic efficiency
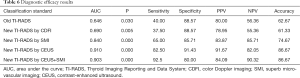
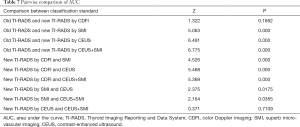
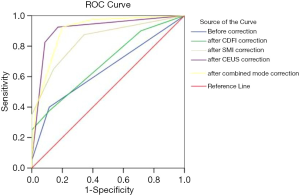
Risk-scoring models were built, and the results of their diagnostic efficacy are summarized in Table 6. The AUC values for the new TI-RADS were 0.690 for CDFI, 0.840 for SMI, 0.910 for CEUS, and 0.903 for SMI + CEUS. CEUS had the highest diagnostic value among the three techniques. Joint inspection using SMI and CEUS provided a certain advantage in terms of sensitivity, yet the overall accuracy was equal to that of CEUS alone. Except for CDFI, the AUCs of the new TI-RADS were significantly higher than those of the old TI-RADS (Table 7 and Figure 3). Among all AUCs for the new TI-RADS, the AUC of CDFI was the lowest. The AUC of CEUS + SMI was higher than that of SMI alone, and was essentially equivalent to CEUS alone. Furthermore, the difference between the AUC for CEUS and SMI was significant.
Full table
Full table
Risk-scoring model
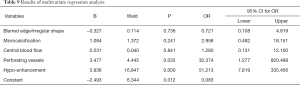
Univariate analysis showed that blur border/spicular/irregular shape, microcalcifications, central blood flow, and perforating vessels detected by SMI, along with nodule hypo-enhancement were related to thyroid cancer (Table 8). Multivariate analysis revealed that perforating vessels and hypo-enhancement were independent risk factors (Table 9).

Full table
Full table
Discussion
Numerous studies have confirmed that vascular imaging in the prediction of benign and malignant thyroid nodules is crucial. In this study, we used the three vascular imaging techniques of CDFI, CEUS, and SMI for the first time to reclassify and correct the subclass of TI-RADS 4 nodules that were included in the study. A risk score model was established to consider the value of flow characteristics in diagnosing thyroid nodules. A comparative study between old and new TI-RADS classifications showed significant differences between these two approaches.
CEUS could reveal blood perfusion in the tissue in real time. In CEUS analysis, high perfusion indicates that a tumor has an extensive microvasculature, whereas a low perfusion suggests a lower degree of microvasculature. Consistent with previous reports, here we showed that malignant nodules mainly displayed hypo-enhancement (13-15), which can be linked to compact fibrosis, functional inefficiency of new vessels, growth heterogeneity, and neovascular damage by tumor cells. Benign nodules typically manifested with hyper-enhancement or iso-enhancement, and the blood supply to these nodules was similar to that in peripheral normal tissue due to the dominance of follicular structures within the benign nodules. The diagnostic accuracy of the new TI-RADS obtained by CEUS correction was improved, particularly with respect to enhanced sensitivity, which was largely consistent with a study by Zhang et al. (16) showing that the malignant rate of nodules below the 4a subclass was decreased, whereas the malignant rate of nodules in subclass 4b and higher was increased compared to the earlier TI-RADS. Although CEUS was associated with high diagnostic accuracy, overlapping enhancement patterns existed between benign and malignant nodules. In the present study, two cases with Hashimoto’s thyroiditis and one with nodular goiter exhibited heterogeneity and hypoenhancement that resulted in the absence of contrast agent in the lesion centers that had large areas of scarring. Meanwhile, seven papillary thyroid cancers (PTC) manifested with hyper-enhancement or iso-enhancement, which could have been due to the following: (I) an abundant blood supply that was available in the tumor itself; and/or (II) high expression levels of proangiogenic factors that could promote angiogenesis and tumor tissue differentiation, resulting in perfusion that is similar to or increased relative to normal thyroid tissue. Such nodules are generally suggestive of an abundance of nourishing vessels, and they tend to grow vigorously with strong invasion. Indeed, Hong et al. (17) reported an increased incidence of capsular invasion or extra-capsular fibrous and adipose tissue invasion, as well as lymph node metastasis in the central cervical region that was associated with hyper-enhancement and iso-enhancement PTC relative to PTC with hypo-enhancement. Although the significance of rim enhancement for benign nodules is unclear, fewer nodules in this study displayed rim enhancement, which could reflect the selective deviation for all nodules in the group that were classified as TI-RADS 4.
SMI could clearly and conveniently display low-velocity blood that CDFI could not, which could be a better depiction of true tumor blood flow and is consistent with findings in a study by Forsberg et al. (18). Our comparison of SMI, CDFI, and CEUS indicated the following: (I) our findings were consistent with results of Phuttharak et al. (19,20) showing different types of vascular distributions in benign and malignant nodules inspected by SMI, wherein peripheral flow and mixed flow were often discovered in benign nodules and central flow was mainly found in malignant nodules; (II) the diagnostic accuracy could be markedly improved by applying SMI compared with the original TI-RADS and in the new TI-RADS using CDFI, although CEUS yielded better results than SMI. This finding demonstrated the value of SMI for diagnosing nodules, even though this approach was not as good as CEUS, which is consistent with conclusions of Lu et al. (21); (III) the accuracy of SMI in diagnosing PTC through identification of perforating vessels was similar to that of CEUS. After assessment of risk factors, perforating vessels and hypo-enhancement were confirmed to be independent risk factors for PTC. Thus, although SMI was inferior to CEUS for the inspection of nodules, SMI nonetheless had advantages for detecting the presence of perforating vessels within the nodules. This finding is in agreement with that by Wu et al. (9), who concluded that SMI better displayed integral vascular networks and was of great value in predicting the nature of such networks. In contrast, CEUS could not detect true perforating vessels, which could be attributed to its high sensitivity to blood flow.
Our results are partly in line with those of Gabriel et al. (22), who pointed out that additional information could be acquired by combining CEUS and SMI. The SMI gray-scale pattern highlights the flow signal by suppressing 2-D tissue information, in turn clarifying the flow branch and direction within the tumor. In comparison, CEUS was superior in reflecting tumor perfusion characteristics through the use contrast agents having distinct flow characteristics that can provide diagnostic benefits. As we indicated in this study, CEUS + SMI had higher sensitivity and lower specificity compared with CEUS or SMI alone, suggesting that the joint model could have a certain rate of misdiagnosis that might be related to an incorrect setting of the joint diagnostic criteria. If the subclasses of the new TI-RADS obtained using CEUS and SMI jointly are not consistent, the diagnostic outcomes may be debated, although when a nodule subclass is downgraded by CEUS alone and upgraded by joint analysis, the nodular diagnosis assigned by CEUS results should be given preference. Conversely, if the subclass is downgraded by SMI and upgraded after joint analysis, we recommend that the joint outcomes be preferred.
Our study has several potential limitations. First, fewer pathological types were explored and the sample size was small, such that some degree of selection bias was unavoidable. This requires more communication and coordination between us and the clinic, and more biopsy before surgery. So that we can collect more cases for research. The size of the selected benign and malignant nodules differed in that the benign nodules were larger, which could have affected the diagnostic efficiency. Meanwhile, the SMI approach only assessed the flow distribution, and this technology is less well-characterized. Furthermore, CEUS only considered the enhancement intensity, and other parameters, such as entry time and extinction time, require additional analysis. Operators must carefully examine the 2-D images to determine whether microcalcification is present to avoid mistaking microcalcification for a flow signal.
Conclusions
In summary, in a comparison of the three ultrasonic flow imaging technologies, CEUS had the highest sensitivity and accuracy for distinguishing benign from malignant thyroid nodules. Although the diagnostic performance of SMI was limited, its sensitivity increased when combined CEUS, indicating that SMI could be a promising tool for diagnosis of thyroid nodule malignancies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-641
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-641
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure forms (available at http://dx.doi.org/10.21037/gs-20-6410. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all study subjects. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by Shanghai East Hospital, Tongji University School of Medicine (No. 102).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk. J Clin Endocrinol Metab 2009;94:1748-51. [Crossref] [PubMed]
- American College of Radiology. Breast imaging reporting and data system, breast imaging atlas. 4th ed. Reston, Va: American College of Radiology, 2003.
- Spira D, Neumeister H, Spira SM, et al. Assessment of tumor vascularity in lung cancer using volume perfusion CT (VPCT) with histopathologic comparison: a further step toward an individualized tumor characterization. J Comput Assist Tomogr 2013;37:15-21. [Crossref] [PubMed]
- Zhan J, Diao XH, Jin JM, et al. Superb Microvascular Imaging—A new vascular detecting ultrasonographic technique for avascular breast masses: Apreliminary study. Eur J Radiol 2016;85:915-21. [Crossref] [PubMed]
- Swinburne NC, Schefflein J, Sakai Y, et al. Machine learning for semi-automated classification of glioblastoma, brain metastasis and central nervous system lymphoma using magnetic resonance advanced imaging. Ann Transl Med 2019;7:232. [Crossref] [PubMed]
- Machado P, Segal S, Lyshchik A, et al. A novel microvascular flow technique: initial results in thyroids. Ultrasound Q 2016;32:67-74. [Crossref] [PubMed]
- Park JY, Lee HJ, Jang HW, et al. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid 2009;19:1257-64. [Crossref] [PubMed]
- Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011;260:892-9. [Crossref] [PubMed]
- Wu C, Zhu L, Chen W, et al. Study on the hemodynamic changes in solid thyroid nodules by ultrasound contrast quantitative analysis. Zhonghua Yi Xue Za Zhi 2015;95:3519-22. [PubMed]
- Zhou Q, Jiang J, Shang X, et al. Correlation of contrast-enhanced ultrasonographic features with microvessel density in papillary thyroid carcinomas. Asian Pac J Cancer Prev 2014;15:7449-52. [Crossref] [PubMed]
- Wendl CM, Janke M, Jung W, et al. Contrast-enhanced ultrasound with perfusion analysis for the identification of malignant and benign tumours of the thyroid gland. Clin Hemorheol Microcirc 2015;63:113-21. [Crossref] [PubMed]
- De Nicola H, Szejnfeld J, Logullo AF, et al. Flow pattern and vascular resistive index as predictors of malignancy risk in thyroid follicular neoplasms. J Ultrasound Med 2005;24:897-904. [Crossref] [PubMed]
- Bartolotta TV, Midiri M, Galia M, et al. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast enhanced ultrasound: initial results. Eur Radiol 2006;16:2234-41. [Crossref] [PubMed]
- Cantisani V, Consorti F, Guerrisi A, et al. Prospective comparative evaluation of quantitative-elastosonography (Q-elastography) and contrast-enhanced ultrasound for the evaluation of thyroid nodules: preliminary experience. Eur J Radiol 2013;82:1892-8. [PubMed]
- Debnam JM, Vu T, Sun J, et al. Vascular flow on doppler sonography may not be a valid characteristic to distinguish colloid nodules from papillary thyroid carcinoma even when accounting for nodular size. Gland Surg 2019;8:461-8. [Crossref] [PubMed]
- Zhang Y, Zhou P, Tian SM, et al. Usefulness of combined use of contrast-enhanced ultrasound and TI-RADS classification for the differentiation of benign from malignant lesions of thyroid nodules. Eur Radiol 2017;27:1527-36. [PubMed]
- Hong YR, Yan CX, Mo GQ, et al. Conventional US, elastography, and contrast enhanced US features of papillary thyroid microcarcinoma predict central compartment lymph node metastases. Sci Rep 2015;5:7748. [PubMed]
- Forsberg F, Machado P, Segal S, et al. Microvascular Blood Flow in the Thyroid:Preliminary Results with a Novel Imaging Technique. Ultrasonics Symposium IEEE 2014;10:2237-40.
- Phuttharak W, Somboonporn C, Hongdomnern G. Diagnostic performance of gray-scale versus combined gray-scale with colour doppler ultrasonography in the diagnosis of malignancy in thyroid nodules. Asian Pac J Cancer Prev 2009;10:759-64. [PubMed]
- Hornung M, Jung EM, Georgieva M, et al. Detection of microvascularization of thyroid carcinomas using linear high resolution contrast-enhanced ultrasonography (CEUS). Clin Hemorheol Microcirc 2012;52:197-203. [Crossref] [PubMed]
- Lu R, Meng Y, Zhang Y, et al. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med Imaging 2017;17:65. [Crossref] [PubMed]
- Gabriel M, Tomczak J, Snoch-Ziółkiewicz M, et al. Comparison of Superb Micro-Vascular Ultrasound Imaging (SMI) and Contrast-Enhanced Ultrasound (CEUS) for Detection of Endoleaks After Endovascular Aneurysm Repair (EVAR). Am J Case Rep 2016;17:43-6. [Crossref] [PubMed]
(English Language Editor: J. Gray)


