
Bilateral pneumothorax in a patient with anaplastic thyroid carcinoma and lung metastasis during lenvatinib therapy: a case report
Introduction
Anaplastic thyroid carcinoma (ATC) accounts for 1.3% to 9.8% of all thyroid cancers, globally (1). The pathogenesis of ATC is not well understood. Patients with ATC may have a history of goiter or co-existing differentiated thyroid cancer or may have no previous history of known thyroid disease before diagnosis (1,2). Patients with ATC present with a rapidly growing thyroid or neck mass that generally invades the surrounding structures and causes compressive symptoms. Additionally, more than 40% of patients with ATC show distant metastasis (2,3). The prognosis of ATC is very poor, and the overall survival of patients with ATC has not improved over the last 50 years despite aggressive radiotherapy and chemotherapy. The treatment strategies for ATC are multimodal, comprising surgery and external beam radiation therapy with chemotherapy for curative intent, if feasible (3). However, the current treatment strategies for ATC have very limited efficacy. Recently, a small series of biomarker-matched targeted therapies has been reported to show promise for the treatment of ATC, suggesting a potential role for checkpoint inhibitors (3).
Lenvatinib, a multi-targeted tyrosine kinase inhibitor, is a novel drug that has shown encouraging antitumor effects on radioactive iodine-refractory papillary thyroid carcinoma. It also appears to be effective for the treatment of ATC; however, the efficacy of the drug requires further evaluation (3-8).
We encountered a case of spontaneous bilateral pneumothorax in a patient with ATC and lung metastasis during lenvatinib therapy. The development of pneumothorax during chemotherapy or targeted therapy is generally a very rare complication, especially in patients with thyroid cancer. To date, there are few reports on the incidence of pneumothorax during lenvatinib therapy (9,10). In this case report, we describe the incidence of bilateral pneumothorax in a patient with ATC and lung metastasis, along with relevant review of literature.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-462).
Case presentation
A 77-year-old man suffering from polyarteritis nodosa, hypertension, and diabetes mellitus, visited the Department of Otolaryngology in February 2019, complaining of a neck swelling since 1 month. He had sore throat, odynophagia, and a globus sensation in the throat. Ultrasonography of the thyroid revealed an irregular, markedly hypoechoic and heterogeneous solid nodular lesion, with macro calcification and mild intranodular vascularity.
Core needle biopsy revealed the lesion to be hypercellular, with high mitotic activity (Ki-67 labelling index 60–70%) and marked pleomorphism. The lesion showed focal positivity for paired box gene 8 (PAX-8) and thyroid transcription factor 1 (TTF-1) and increased p53 expression upon immunohistochemical staining. The final pathological diagnosis was ATC.
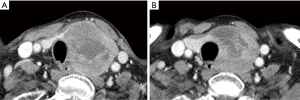
Contrast-enhanced computed tomography (CT) revealed a mass of dimensions 6.1 cm × 4.8 cm × 4.7 cm in the left lobe of the thyroid, abutting the trachea and esophagus (Figure 1A). Multiple metastatic masses were also observed in the parenchyma of both the lungs. Treatment with lenvatinib was planned during a multi-disciplinary team meeting. The patient tolerated treatment with 24 mg lenvatinib and did not show any severe side effects. Neck CT was performed on day 36 of lenvatinib therapy, which showed a decrease in the volume of the primary thyroid tumor (Figure 1B).
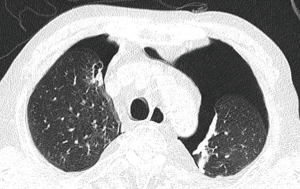
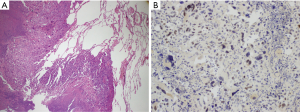
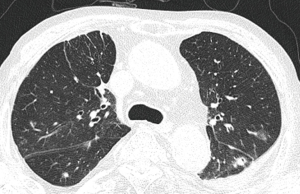
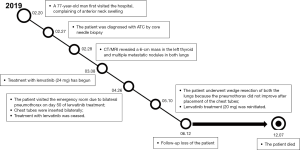
The patient visited the emergency room complaining of dyspnea on day 50 of lenvatinib treatment, and his blood oxygen saturation on ambient air was 82%. Chest CT showed the incidence of bilateral pneumothorax (Figure 2). Chest tubes were bilaterally inserted for the pneumothorax, and treatment with lenvatinib was ceased. However, the pneumothorax did not improve after placement of the chest tubes. The patient underwent wedge resection of both the lungs, and pathological examination revealed metastatic lesions of ATC (Figure 3). The pneumothorax stabilized after bilateral lung operation (Figure 4). Lenvatinib treatment was reinitiated at a reduced dose of 20 mg, and the growth of the tumor appeared to be stationary. We could not follow up on the patient one month after taking a reduced dose of lenvatinib. We have heard from the patient’s family that the patient died due to dyspnea and general weakness in December 2019. The timeline of the whole process of the patient is outlined in Figure 5.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
Discussion
Lenvatinib is an active multi-targeted tyrosine kinase inhibitor that acts on vascular endothelial growth factor (VEGF) receptors 1–3, platelet-derived growth factor receptor-α, fibroblast growth factor (FGF) receptors 1–4, platelet-derived growth factor receptor-α, the RET proto-oncogene, and the KIT proto-oncogene receptor tyrosine (6,7). Lenvatinib does not significantly inhibit the proliferation of tumor cells, but affects the migration and invasion of tumor cells, owing to its anti-angiogenic activity. Tahara and coworkers reported that lenvatinib has manageable toxicities following dose adjustments and clinical activity in patients with ATC, and the objective response rate reported in their study was 24% (4). The common adverse events of lenvatinib include hypertension, diarrhea, fatigue, appetite suppression, weight loss, and nausea (4,7,8).
The present case involved spontaneous bilateral pneumothorax that developed as a side effect of lenvatinib therapy in a patient with ATC and lung metastasis. In the absence of iatrogenic causes, the development of spontaneous pneumothorax during chemotherapy has been most frequently reported in patients with lung cancer. The development of spontaneous pneumothorax in patients with thyroid cancer and lung metastasis is a very rare sequela of lenvatinib therapy for thyroid cancer (11). To date, there have been only two reports of the development of spontaneous pneumothorax during lenvatinib therapy for patients with ATC and lung metastasis (9,10).
The etiologies of spontaneous pneumothorax during targeted therapy or chemotherapy for lung metastasis are yet to be completely understood. Various causes have been suggested for the development of spontaneous pneumothorax from lung metastasis, including the rupture of necrotic tumor nodules, necrosis of subpleural metastases, direct pleural invasion by the tumor, and the involvement of the check valve mechanism of the tumor nodules at the periphery of the lung (12-15).
In this case, the initial chest CT before lenvatinib therapy did not reveal any subpleural bulla, pleural invasion, or pneumothorax. However, the serial follow-up CT during lenvatinib therapy revealed the presence of multiple thin-walled cavitations around the metastatic lesions of the lung. We assume that the metastatic lesions and the cavitations were related to tumor necrosis following lenvatinib therapy, which resulted in air leakage and the occurrence of the bilateral pneumothorax.
The patient underwent video-assisted thoracoscopic surgery (VATS) in both the lungs because the pneumothorax continued to aggravate even after the insertion of chest tubes. The pneumothorax stabilized after bilateral lung resection, and the growth of the tumor also appeared to be stationary following the reinitiation of lenvatinib. Surgical intervention should thus be considered if the pneumothorax persists even after the insertion of chest tubes. Successful and well-tolerated VATS has several advantages in that it is less painful and allows better postoperative pulmonary gas exchange than open thoracotomy and surgical or chemical pleurodesis (16).
There are some limitations to this study. It is a case report. Therefore, it is difficult to evaluate the whole characteristics of pneumothorax in lenvatinib treatment for thyroid cancer. Also, we lost the patient during the treatment. However, it may be noteworthy that this study suggested the possibility and mechanism of spontaneous pneumothorax in lenvatinib therapy.
In conclusion, clinicians need to be aware that the development of pneumothorax is a possibility during the treatment of patients with lenvatinib for ATC and lung metastasis. It is therefore necessary to closely monitor the patient for the early detection of pneumothorax.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-462
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-462). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 2010;22:486-97. [Crossref] [PubMed]
- Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012;22:1104-39. [Crossref] [PubMed]
- Cabanillas ME, Zafereo M, Gunn GB, et al. Anaplastic Thyroid Carcinoma: Treatment in the Age of Molecular Targeted Therapy. J Oncol Pract 2016;12:511-8. [Crossref] [PubMed]
- Tahara M, Kiyota N, Yamazaki T, et al. Lenvatinib for Anaplastic Thyroid Cancer. Front Oncol 2017;7:25. [Crossref] [PubMed]
- Takahashi S, Kiyota N, Yamazaki T, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol 2019;15:717-26. [PubMed]
- Iyer PC, Dadu R, Ferrarotto R, et al. Real-World Experience with Targeted Therapy for the Treatment of Anaplastic Thyroid Carcinoma. Thyroid 2018;28:79-87. [PubMed]
- Glen H, Mason S, Patel H, et al. E7080, a multi-targeted tyrosine kinase inhibitor suppresses tumor cell migration and invasion. BMC Cancer 2011;11:309. [Crossref] [PubMed]
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. [Crossref] [PubMed]
- Yamazaki H, Iwasaki H, Yamashita T, et al. A Case of Pneumothorax after Treatment with Lenvatinib for Anaplastic Thyroid Cancer with Lung Metastasis. Case Rep Endocrinol 2018;2018:7875929. [Crossref] [PubMed]
- Kazzaz FI, Cabanillas ME, Bashoura L, et al. Bilateral spontaneous pneumothoraces in anaplastic thyroid cancer. Respir Med Case Rep 2019;26:197-9. [Crossref] [PubMed]
- Berdelou A, Borget I, Godbert Y, et al. Lenvatinib for the Treatment of Radioiodine-Refractory Thyroid Cancer in Real-Life Practice. Thyroid 2017. Epub ahead of print. [Crossref] [PubMed]
- Lee MJ, Kim EK, Kim MJ, et al. Spontaneous pneumothorax in metastatic thyroid papillary carcinoma. J Clin Oncol 2007;25:2616-8. [Crossref] [PubMed]
- Maniwa T, Nakagawa K, Isaka M, et al. Pneumothorax associated with treatment for pulmonary malignancy. Interact Cardiovasc Thorac Surg 2011;13:257-61. [Crossref] [PubMed]
- Mori M, Nakagawa M, Fujikawa T, et al. Simultaneous bilateral spontaneous pneumothorax observed during the administration of gefitinib for lung adenocarcinoma with multiple lung metastases. Intern Med 2005;44:862-4. [Crossref] [PubMed]
- Zhang Y, Yang H, Zhao M, et al. Bilateral pneumothorax after bevacizumab-containing chemotherapy in fibrosarcoma. J Thorac Dis 2012;4:229-31. [PubMed]
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. [Crossref] [PubMed]


