
BRAFV600E mutation combined with American College of Radiology thyroid imaging report and data system significantly changes surgical resection rate and risk of malignancy in thyroid cytopathology practice
Introduction
Fine needle aspiration (FNA) biopsy of the thyroid gland has been developed as a primary procedure for evaluating patients with thyroid nodules (1). Ultrasonography is recommended as an essential method in malignancy risk stratification for thyroid nodules and for decisions concerning FNA of thyroid nodules (2). The American College of Radiology (ACR) has created an ultrasound malignancy risk stratification system for thyroid nodules named the ACR Thyroid Imaging Reporting and Data System (TI-RADS), in which the malignancy risk is stratified by suspicious ultrasound features (3). The ACR TI-RADS has demonstrated some capacity to select high risk-patients for FNA biopsy, and a combination of cytopathology and TI-RADS has shown a considerable ability to differentiate high-risk thyroid nodules (4,5).
The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was developed by the American National Cancer Institute in 2009 to standardize the interpretation of the FNA cytology (FNAC) results (6). The TBSRTC system has been widely used and helps reduce unnecessary surgeries (7). A few specific output measurements, including frequency of thyroid FNA samples, resection rate (RR), and risk of malignancy (ROM) in each diagnostic category, serve as quality control criteria (1,6).
The utility of molecular techniques for thyroid nodules has wide application, and it may enhance the diagnostic value of FNA (8). BRAFV600E is the most specific single molecular diagnostic test for thyroid lesions (9), and all thyroid tumors which contain BRAFV600E mutation are considered malignant (10).
In this study, we evaluated the diagnostic value of ACR TI-RADS and BRAFV600E mutation analysis based on the corresponding FNAC and surgical histopathology results. We calculated the RR and ROM for TBSRTC before and after the implementation of ACR TI-RADS and BRAFV600E mutation analysis at our institute. This is a validation study of the diagnostic performance of ACR TI-RADS, FNAC, and BRAFV600E molecular test analysis in a Chinese population. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/gs-20-535).
Methods
Subjects
This was a retrospective study. The study protocol was reviewed and approved by the Institutional Review Board of Jiangsu Institute of Nuclear Medicine (IRB No. YL201601) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all patients included in the study. Incidental microcarcinomas detected in surgical specimens were excluded from statistical analysis.
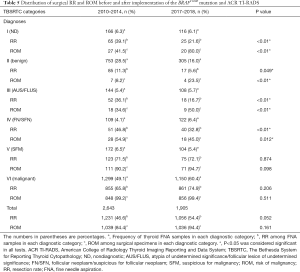
From February 1, 2010, to February 1, 2014, a total of 2,643 consecutive thyroid nodules from 2,399 patients (688 men and 1,711 women) underwent preoperative ultrasound-guided or palpation-guided FNA biopsies. Thyroid ultrasound records were available for all patients who were included in this study. During this period, all thyroid nodules did not undergo ACR TI-RADS stratification and the BRAFV600E mutation test. The average age was 44.3±12.5 years old, and the mean diameter was 1.4±0.9 cm (Table 1). The cytological reports were adjusted with the 2009 TBSRTC system and classified into six categories. Our institution has used the TI-RADS stratification and BRAFV600E mutation analysis as a routine procedure since January 2016. Between February 1, 2017, and July 31, 2018, a total of 2,167 consecutive thyroid nodules were found from 2,011 patients who underwent preoperative ultrasound-guided FNA biopsies. During this period, nodules that met the following criteria were included in this survey: (I) thyroid cytology based on the six-tiered 2009 TBSRTC system; (II) availability of BRAFV600E mutation analysis; (III) an ultrasound examination of the nodules classified by the five-tiered ACR TI-RADS. Finally, a total of 1,905 thyroid nodules from 1,837 patients (501 men and 1,336 women) were enrolled; 262 out of 2,167 nodules were excluded from the survey because patients refused to undergo molecular tests, or the ultrasound contained only a descriptive report without ACR TI-RADS grading. The average age was 49.5±12.8 years old, and the mean diameter was 1.1±0.7 cm. The difference in average age and nodule diameter between the periods spanning 2010 to 2014 and 2017 to 2018 was significant (P<0.01) (Table 1).

Full table
Three cytopathologists specializing in thyroid pathology shared the work, and each sample was assessed by two cytopathologists. Our institution has adopted the original TBSRTC system to reassess the cytological reports. This system uses the following six-tiers for risk categorization (6): nondiagnostic (ND, I), benign (II), atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS, III), follicular neoplasm/suspicious for follicular neoplasm (FN/SFN, IV), suspicious for malignancy (SFM, V); and malignant (VI) (1). Our institution has adopted the borderline tumor category introduced by the latest World Health Organization classification of endocrine organs (11) in routine histopathology practice, which includes follicular tumor of uncertain malignant potential (FT-UMP), well-differentiated tumor of uncertain malignant potential (WDT-UMP), and non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP).
Criteria for FNA
The FNA criteria mainly included enlarging nodule size, abnormal serological tests, suspicious ultrasound features, and clinician preference. After the adoption of ACR TI-RADS, ultrasound evaluation as TI-RADS 5 was recommended for FNA biopsy. FNA is not recommended for TI-RADS 1 and TI-RADS 2 thyroid nodules because of the low potential for malignancy (3). TI-RADS 3 nodules measuring ≥2.5 cm and TI-RADS 4 nodules measuring ≥1.5 cm were recommended for FNA biopsy (12), other factors indicating FNA included patients who had been exposed to previous radiation to the neck, those with a family history of thyroid cancer, and those with nodule growth observed during surveillance (8).
Criteria for surgical treatment
Thyroid surgery was recommended to all FNA-BRAFV600E mutation-positive patients regardless of the cytological reports. For BRAFV600E mutation-negative cases, patients with SFM (V) or malignant (VI) were recommended for surgeries. Repeated ultrasound-guided FNA or follow-up ultrasound was recommended for the patients with ND (I) and AUS/FLUS (III) reports. FN/SFN (IV) with a TI-RADS 4 or TI-RADS 5 ultrasound report would be recommended for surgical treatment, otherwise those with a TI-RADS 2 or TI-RADS 3 score were recommended to undergo active surveillance. Patients with benign (II) reports were commonly recommended for ultrasound follow-up except for those with hyperfunctioning goiter, pressure symptoms, or if the enlarged nodule would influence appearance.
Ultrasonography examination
Ultrasound examinations and ACR TI-RADS were conducted as described previously (13). TI-RADS, consisting of a five-tiered risk classification based on suspicious ultrasound features [2,3], has been used to classify each thyroid nodule at our institution since March 2015. All ultrasound examinations were performed using a 5–12 MHz linear-array probe (iU22, Philips Healthcare, Bothell, WA, USA) by 1 of 10 radiologists who had 3 to 24 years of experience in thyroid imaging (13).
FNA procedure and BRAFV600E detection
Ultrasound-guided FNA was performed for nodules with suspicious features by 1 of 5 certified radiologists, using a previously described procedure (13,14). The procedure was conducted in the hospital outpatient setting after informed consent was obtained from the patient. Thyroid FNA samples were collected under ultrasound guidance with a 23-gauge needle attached to a 5-mL syringe. Two to four passes were made for each thyroid nodule. The slides were wet-fixed in 95% ethanol and then sent for hematoxylin and eosin staining. On-site cytological assessment was not available. The residual biopsy samples in the syringe were expelled for molecular testing after cytological preparation. The wash-out of the needle tip was pipetted into a microcentrifuge tube for molecular testing. DNA was extracted using the QIAamp DNA Micro kit (Qiagen) according to the manufacturer’s instructions. The method for BRAFV600E mutation analysis has been described previously (13,15).
Statistical analysis
Statistical analysis was performed with SPSS statistical software, version 23.0 (IBM, Armonk, NY, USA). Two-tailed chi-square (χ2) test or Fisher’s exact test was used to evaluate potential differences in the categorical variables between groups. Two-sample t-test was adopted for the comparison of variations. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy (AC) of ACR TI-RADS, BRAFV600E mutation, and the combination of the two methods were evaluated. For all tests, a P value <0.05 was considered an indication of a significant difference.
Results
Test for BRAFV600E mutation
As shown in Figure 1, The percentages of BRAFV600E mutation rates in 1,905 FNA samples, according to the TBSRTC I–VI categories, were 12.9%, 1.3%, 5.6%, 2.6%, 79.8%, and 82.2%, respectively. A total of 1,036 nodules out of 1,905 had final histological diagnoses, and 978 were found to be malignant. Of the 1,036 surgical-treated specimens, borderline tumors accounted for a minimal proportion in each TBSRTC category, with none being ND (I) or benign (II), two (2/18) being AUS/FLUS (III), five (5/40) being FN/SFN (IV), one (1/75) being SFM (V), and two being (2/861) malignant (VI) (Figure 1). The 25 nodules with TBSRTC I–III cytology reports and positive mutation results were recommended for surgical treatment. One patient refused surgery, and 24 nodules proved to be papillary thyroid carcinomas (PTCs). Another 504 nodules classified as TBSRTC I–III cytology reports, and negative for the BRAFV600E mutation, were recommended for surveillance every 6 months using ultrasound with or without repeated FNA that did not exhibit any changes over time.
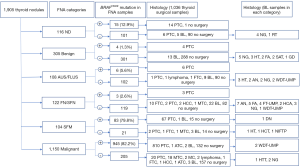
BRAFV600E mutation was found in 1,028 nodules with SFM (V) or malignant (VI) cytology. In all, 881 of these nodules had histopathological results, including 877 PTCs, one anaplastic carcinoma, and three benign/borderline lesions. Of the three BRAFV600E mutation-positive with histologically proven “no cancer” lesions, the FNAC was SFM (V) in one and malignant (VI) in two. On specimen pathology, the nodule with SFM (V) showed extensive sclerosis and calcifications, and thus led to a diagnosis of degenerative nodule (DN) with calcification. The two nodules with malignant (VI) cytology were completely replaced by cystic and regressive changes. Deeper sections of these two specimens revealed micro-focus suspicion of PTC, leading to a diagnosis of WDT-UMP. It has been reported that ultrasound-guided FNA may lead to the complete vanishing of thyroid nodules, rendering final diagnosis upon surgical histopathology difficult or impossible (16,17).
Of the 1,036 surgical specimens that were assessed, 908 were positive for the BRAFV600E mutation, and 128 were negative. Thus, the RR of BRAFV600E mutation-positive nodules was 87.6% (908/1,036). The cancer risk in nodules with BRAFV600E mutation was 99.7% (905/908) according to the histological results.
As shown in Table 2, among the 1,036 surgical specimens, PTCs account for a relatively high percentage in each TBSRTC category (80.0% in ND, 23.5% in benign, 38.9% in AUS/FLUS, 12.5% in FN/SFN, 92.0% in SFM, and 96.4% in malignant). BRAFV600E mutation was identified in 96.7% of PTCs based on FNA-surgery samples. The BRAFV600E mutation incidence in PTCs according to the TBSRTC was 70.0% for ND, 100% for benign, 85.7% for AUS/FLUS, 60.0% for FN/SFN, 97.1% for SFM, and 97.6% for malignant. The high rate of BRAFV600E mutation in histologically proven PTCs may be due to the BRAFV600E mutation serving as an independent marker of aggressiveness of PTC and affecting the frequency of lymph node metastases (18,19). Therefore, clinicians tend to prioritize patients with FNA-BRAFV600E mutation for surgery.

Full table
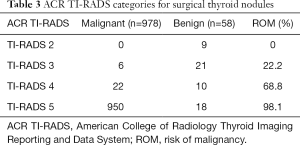
Malignancy risk for each ACR TI-RADS category according to histopathological results
The malignant rates of the 1,036 surgical nodules classified as TI-RADS 2, 3, 4, 5 were 0, 22.2%, 68.8%, and 98.1%, respectively, according to the postoperative histopathology (Table 3).
Full table
Diagnostic performance of BRAFV600E and ACR TI-RADS
The diagnostic performance, including sensitivity, specificity, PPV, NPV, and accuracy (AC), according to BRAFV600E mutation analysis, ACR TI-RADS, and the combination of both are summarized in Table 4. On applying a higher threshold for positivity (ACR TI-RADS 5), the sensitivity, specificity, PPV, NPV, and AC for malignancy were 97.1%, 68.9%, 98.1%, 58.8%, and 95.5%, respectively. The combination of TI-RADS 5 and BRAFV600E mutation analysis showed the best AC (97.5%) and significantly increased the specificity (94.8%) compared to the TI-RADS 5 alone (68.9%).

Full table
Variations of RR and ROM after the inclusion of BRAFV600E mutation test and ACR TI-RADS
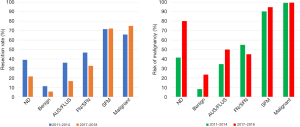
The distribution of surgical RR and ROM before and after the implementation of the ACR TI-RADS and BRAFV600E mutation test is displayed in Table 5. A total of 2,643 nodules underwent FNA biopsies from February 1, 2010, to February 1, 2014, before the ACR TI-RADS and BRAFV600E mutation analysis were implemented. In comparison, BRAFV600E mutation analysis and ACR TI-RADS reports were available for 1,905 FNA samples which were obtained between February 2017, and July 2018. Inclusion of the ACR TI-RADS and BRAFV600E mutation analysis significantly decreased RR and increased ROM for TBSRTC I–III categories (P<0.05) (Figure 2). The most considerable increase in the ROM (41.5% vs. 80%) was observed for the ND (I) category. A slight decrease in the ROM was found for the FN/SFN (IV) category (54.9% vs. 45.0%). No significant difference was found in the SFM (V) and malignant (VI) categories (Table 5 and Figure 2).
Full table
Discussion
The most common FNA diagnosis rendered in our practice was malignant (VI), which was high at 49.1% (1,299/2,643) from February, 2010 to February, 2014, and even higher at 60.4% (1,150/1,905) from February, 2017 to July, 2018. This is an extremely high proportion of malignant FNA diagnoses in comparison with the average of 7.4% found in 11 reports summarized by Sheffield et al. (20). We considered three explanations for the relatively high rate of the malignant category. First, there has been a marked increase in thyroid operations in our hospital. Nearly 4,000 thyroid operations were performed in 2019, whereas approximately 1,500 operations were taken per year from 2000 to 2010. Thyroid clinics have been expanded over the past decade. The explosive growth of ultrasound-suspected thyroid nodules and surgeries may force Chinese endocrinologists to concentrate more on patients with a higher ROM, and postpone treatment of biologically more indolent borderline or precursor tumors to active surveillance, which, at the same time, can help reduce the workload of medical staff. This approach also resulted in achieving an extremely high overall ROM at 94.4% (1,036/1,056, from 2017 to 2018), and thus may be an ideal model on how to eliminate unnecessary diagnostic surgery and reduce the overtreatment of indolent thyroid tumors. The high ROM of surgical-treated thyroid nodules is not unusual in Chinese practice (21,22), which is very different from Western countries, where diagnostic surgeries to benign or borderline tumors are common and accepted practice (23,24). The second reason was that all thyroid nodules in the study were classified by ultrasound, and FNAs were performed only when necessary. The third contributing factor might be the fact that our hospital is a thyroid cancer center, which also provides radioactive iodine (RAI) 131 treatment. Thus, the prevalence of thyroid cancer may be higher at our center compared to that of hospitals in a community hospital setting.
The malignancy rate of each category was found to be much higher than that initially recommended by the TBSRTC, especially in the TBSRTC I, II, and III categories even before the implementation of the ACR TI-RADS and BRAFV600E mutation analysis (41.5% vs. 5–10%, 8.2% vs. 0–3%, 34.6% vs. 10–30% respectively) (6), indicating that more conservative clinical management or strictly triage patients for surgery was favored. These higher risks of malignancy were also similarly achieved in other Asian practices using the same TBSRTC system (22,25-27). In a recent meta-analysis, Vuong et al. analyzed a total of 38 studies with 145,066 fine-needle aspirations and found that Asian series had a significantly higher ROM in TBSRTC I, II, and III categories when compared with Western practice (26.5% vs. 13.2%, 13.8% vs. 4.1%, 45.9% vs. 21.5%) (28). The high ROM in benign or indeterminate categories indicates that decisions to carry out thyroidectomy are thorough in Asian practice (25).
The main aim of thyroid FNA is to assist clinicians in their decisions on the patient’s management (29). Benign (II), SFM (V), and malignant (VI) categories have “sufficient for decision” risks of malignancy in our practice (8.2%, 90.2%, and 99.2%, respectively). Subsequently, cytopathological diagnoses in these three categories should give clinicians clear treatment suggestions. The problematic categories of ND (I), AUS/FLUS (III), and FN/SFN (IV) indicate risks of malignancy that are in the indeterminate range of 34.6% to 54.9%, and therefore represent a dilemma in treatment decision-making.
The ACR TI-RADS categories, which include, benign (TI-RADS 1, TI-RADS 2), mildly suspicious (TI-RADS 3), moderately SFM (TI-RADS 4), or highly SFM (TI-RADS 5) (3), can accurately and efficiently predict malignancy risks according to the number of suspicious ultrasound features (3). The BRAFV600E mutation is highly specific to PTC, and false-positive tests are rarely reported, making it a powerful adjunct for PTC (30).
Therefore, we have adopted the ACR TI-RADS classification and BRAFV600E mutation analysis, together with FNAC, as a routine procedure, intending further to assess the risk of the patients with TBSRTC I, III, and IV reports. In our study, the ROM is inclined to increase with advancing TI-RADS category (Table 3). High-risk ultrasound features (TI-RADS 5) did show a good performance in predicting malignancy (98.1%). The combination of TI-RADS 5 and BRAFV600E mutation reached the best diagnostic efficiency (sensitivity 97.7%, specificity 94.8%, PPV 99.6%). It is apparent that after the implementation of ACR TI-RADS and the BRAFV600E mutation analysis, the RRs of TBSRTC I and III categories showed significant decreases (39.1% vs. 21.6% and 36.1% vs. 16.7%, respectively). In contrast, the risks of malignancy of TBSRTC I and III categories indicated substantial increases (41.5% vs. 80.0% and 34.6% vs. 50.0%, respectively). The ROM of thyroid nodules with the ND (I) category showed the most significant increase from 41.5% to 80.0% after the inclusion of the ACR TI-RADS and BRAFV600E mutation analysis, indicating such a combination of molecular test with ultrasound features could exhibit high diagnostic value in predicting malignancy and help prevent unnecessary diagnostic surgeries.
Repeated ultrasound-guided FNA, or follow-up ultrasound was recommended for the patients with ND (I) and AUS/FLUS (III) reports (6). Some authors recommended routine repeated FNA or selective FNA to avoid false-negative results (31,32). However, this practice may result in unnecessary FNA and have low cost-effectiveness (33). There are very few repeated FNAs in our practice. Nodules with BRAFV600E mutation-positive results will be directly recommended for surgery regardless of the cytological reports. For indeterminate nodules with BRAFV600E mutation-negative results, after excluding clinical high-risk factors, repeated FNA would only be considered for those who have demonstrated three or more suspicious ultrasound features (33,34).
Some limitations to our study should be addressed. Our study was a single thyroid cancer center study, and most of the thyroid nodules in our series had a TBSRTC-VI FNA diagnosis. PTC accounts for a majority of malignant diagnoses (95.6%, 935/978, from 2017 to 2018). As a result, some selection bias was unavoidable. Furthermore, BRAFV600E mutation has an especially exclusionary role for PTC, whereas the absence of BRAFV600E mutation does not rule out the presence of PTC (35). Classic PTC commonly exhibits high-suspicion ultrasound patterns (TI-RADS 5). However, follicular carcinoma and follicular variant PTC are present in relatively large proportions among malignant tumors with low-suspicion ultrasound features (3,36,37). The high specificity of TI-RADS 5 and BRAFV600E mutation for PTC, to some extent, explained why the implementation of ACR TI-RADS and BRAFV600E mutation did not increase the ROM for the FN/SFN (IV) category since PTCs account for a small percentage of this category (12.5%). In contrast, PTCs account for a relatively high proportion of the ND (80%) and AUS/FLUS categories (38.9%).
The above factors provide ACR TI-RADS and BRAFV600E mutation analysis with the powerful ability to efficiently identify PTC in each TBSRTC category but might have contributed to an overestimation of our findings. Nevertheless, collecting accurate data from local institutional cytological/histopathological surveys of risk for malignancy in each cytological subcategory remains a worthwhile endeavor, as there is wide inter-institutional variation in the ROM across the various categories (5,28). Moreover, an individualized risk prediction model to accurately assess the cancer risk of patients with cytologically indeterminate thyroid nodules is helpful for clinicians to more reliably select patients for surgery (38). A final limitation of our study is that 10 certified radiologists performed the ultrasound examination, and thus intra- and interobserver variability in interpreting the ACR TI-RADS reports might have been present (39).
Conclusions
ACR TI-RADS, BRAFV600E mutation, and cytological diagnoses, can assist in improving the prediction of thyroid nodule malignancy, especially in the ND (I) and AUS/FLUS (III) categories. Clinicians can apply different management strategies in their use of TBSRTC, and formulating a more individualized treatment schedule may help in making informed decisions on indeterminate nodules and improving diagnostic performance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kennichi Kakudo) for the series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” published in Gland Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-535
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-535
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-535). The series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed and approved by the Institutional Review Board of Jiangsu Institute of Nuclear Medicine (IRB No. YL201601). Informed consent was obtained from all patients included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017;27:1341-46. [Crossref] [PubMed]
- Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab 2009;94:1748-51. [Crossref] [PubMed]
- Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017;14:587-95. [Crossref] [PubMed]
- Grani G, Lamartina L, Ascoli V, et al. Reducing the Number of Unnecessary Thyroid Biopsies While Improving Diagnostic Accuracy: Toward the "Right" TIRADS. J Clin Endocrinol Metab 2019;104:95-102. [Crossref] [PubMed]
- Lauria Pantano A, Maddaloni E, Briganti SI, et al. Differences between ATA, AACE/ACE/AME and ACR TI-RADS ultrasound classifications performance in identifying cytological high-risk thyroid nodules. Eur J Endocrinol 2018;178:595-603. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009;19:1159-65. [Crossref] [PubMed]
- Bongiovanni M, Spitale A, Faquin WC, et al. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol 2012;56:333-39. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 2009;94:2092-98. [Crossref] [PubMed]
- Jinih M, Foley N, Osho O, et al. BRAF(V600E) mutation as a predictor of thyroid malignancy in indeterminate nodules: A systematic review and meta-analysis. Eur J Surg Oncol 2017;43:1219-27. [Crossref] [PubMed]
- Lloyd RV, Osamura RY, Klöppel GK, et al. WHO Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press, 2017.
- Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011;260:892-9. [Crossref] [PubMed]
- Wu H, Zhang B, Cai G, et al. American College of Radiology thyroid imaging report and data system combined with K-RAS mutation improves the management of cytologically indeterminate thyroid nodules. PLoS One 2019;14:e0219383. [Crossref] [PubMed]
- Zhu Y, Dai J, Lin X, et al. Fine needle aspiration of thyroid nodules: experience in a Chinese population. J Basic Clin Med 2015;4:65-9.
- Zhang YZ, Xu T, Cui D, et al. Value of TIRADS, BSRTC and FNA-BRAF V600E mutation analysis in differentiating high-risk thyroid nodules. Sci Rep 2015;5:16927. [Crossref] [PubMed]
- Eze OP, Cai G, Baloch ZW, et al. Vanishing thyroid tumors: a diagnostic dilemma after ultrasonography-guided fine-needle aspiration. Thyroid 2013;23:194-200. [Crossref] [PubMed]
- Bhatia P, Deniwar A, Mohamed HE, et al. Vanishing tumors of thyroid: histological variations after fine needle aspiration. Gland Surg 2016;5:270-7. [Crossref] [PubMed]
- Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943-9. [Crossref] [PubMed]
- Kim SJ, Lee KE, Myong JP, et al. BRAF V600E mutation is associated with tumor aggressiveness in papillary thyroid cancer. World J Surg 2012;36:310-7. [Crossref] [PubMed]
- Sheffield BS, Masoudi H, Walker B, et al. Preoperative diagnosis of thyroid nodules using the Bethesda System for Reporting Thyroid Cytopathology: a comprehensive review and meta-analysis. Expert Rev Endocrinol Metab 2014;9:97-110. [Crossref] [PubMed]
- Liu Z, Liu D, Ma B, et al. History and Practice of Thyroid Fine-Needle Aspiration in China, Based on Retrospective Study of the Practice in Shandong University Qilu Hospital. J Pathol Transl Med 2017;51:528-32. [Crossref] [PubMed]
- Ke J, Jianyong L, Ying L, et al. The use of The Bethesda System for Reporting Thyroid Cytopathology in a Chinese population: An analysis of 13 351 specimens. Diagn Cytopathol 2019;47:876-80. [Crossref] [PubMed]
- Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2017;27:481-3. [Crossref] [PubMed]
- Ferris RL, Nikiforov Y, Terris D, et al. AHNS Series: Do you know your guidelines? AHNS Endocrine Section Consensus Statement: State-of-the-art thyroid surgical recommendations in the era of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Head Neck 2018;40:1881-8. [Crossref] [PubMed]
- Kakudo K, Higuchi M, Hirokawa M, et al. Thyroid FNA cytology in Asian practice-Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017;28:455-66. [Crossref] [PubMed]
- Bychkov A, Kakudo K, Hong S. Current Practices of Thyroid Fine-Needle Aspiration in Asia: A Missing Voice. J Pathol Transl Med 2017;51:517-20. [Crossref] [PubMed]
- Jung CK, Hong S, Bychkov A, et al. The Use of Fine-Needle Aspiration (FNA) Cytology in Patients with Thyroid Nodules in Asia: A Brief Overview of Studies from the Working Group of Asian Thyroid FNA Cytology. J Pathol Transl Med 2017;51:571-78. [Crossref] [PubMed]
- Vuong HG, Ngo HTT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: A systematic review and meta-analysis. Cancer Cytopathol 2020;128:238-49. [Crossref] [PubMed]
- Krauss EA, Mahon M, Fede JM, et al. Application of the Bethesda Classification for Thyroid Fine-Needle Aspiration: Institutional Experience and Meta-analysis. Arch Pathol Lab Med 2016;140:1121-31. [Crossref] [PubMed]
- Fnais N, Soobiah C, Al-Qahtani K, et al. Diagnostic value of fine needle aspiration BRAF(V600E) mutation analysis in papillary thyroid cancer: a systematic review and meta-analysis. Hum Pathol 2015;46:1443-54. [Crossref] [PubMed]
- Menéndez Torre E, Pineda Arribas J, Martínez de Esteban JP, et al. Value of repeated fine needle aspiration cytology in patients with nodular goiter. Acta Cytol 2007;51:850-2. [Crossref] [PubMed]
- Orlandi A, Puscar A, Capriata E, et al. Repeated fine-needle aspiration of the thyroid in benign nodular thyroid disease: critical evaluation of long-term follow-up. Thyroid 2005;15:274-78. [Crossref] [PubMed]
- Moon HJ, Kim EK, Yoon JH, et al. Malignancy risk stratification in thyroid nodules with nondiagnostic results at cytologic examination: combination of thyroid imaging reporting and data system and the Bethesda System. Radiology 2015;274:287-95. [Crossref] [PubMed]
- Khoncarly SM, Tamarkin SW, McHenry CR. Can ultrasound be used to predict malignancy in patients with a thyroid nodule and an indeterminate fine-needle aspiration biopsy. Surgery 2014;156:967-70. [Crossref] [PubMed]
- Rossi M, Buratto M, Bruni S, et al. Role of ultrasonographic/clinical profile, cytology, and BRAF V600E mutation evaluation in thyroid nodule screening for malignancy: a prospective study. J Clin Endocrinol Metab 2012;97:2354-61. [Crossref] [PubMed]
- Hong MJ, Na DG, Baek JH, et al. Cytology-Ultrasonography Risk-Stratification Scoring System Based on Fine-Needle Aspiration Cytology and the Korean-Thyroid Imaging Reporting and Data System. Thyroid 2017;27:953-59. [Crossref] [PubMed]
- Shin JH, Baek JH, Chung J, et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2016;17:370-95. [Crossref] [PubMed]
- Öcal B, Korkmaz MH, Yilmazer D, et al. The Malignancy Risk Assessment of Cytologically Indeterminate Thyroid Nodules Improves Markedly by Using a Predictive Model. Eur Thyroid J 2019;8:83-9. [Crossref] [PubMed]
- Hoang JK, Middleton WD, Farjat AE, et al. Interobserver Variability of Sonographic Features Used in the American College of Radiology Thyroid Imaging Reporting and Data System. AJR Am J Roentgenol 2018;211:162-67. [Crossref] [PubMed]
(English Language Editor: J. Gray)


