
Predictive factors of lateral lymph node metastasis in conventional papillary thyroid carcinoma
Introduction
The incidence rate of thyroid cancer, predominantly PTC, continues to increase rapidly (1,2). Compared with other subtypes of thyroid cancer, PTC prefers to metastasize through lymphatic channel. Many studies have shown that LNM in PTC is a significant risk factor of local recurrence and distant metastasis, which may decrease the patient survival rate (3-5). And the percentage of lateral compartment metastasis in LNM of PTC ranges from 28.8–80.6% (6,7). Traditionally, lymphatic metastasis first takes place in the central compartment and subsequently invades the lateral sections by following the anatomic lymphatic channels (8). However, skip metastasis, which is defined as shift of metastasis to the lateral neck without central compartment metastasis, can sometimes occur (9). For treatment, although radioactive iodine therapy and systemic therapy are important elements for a multifaceted treatment of PTC, surgery is always the essential part and top-priority treatment of PTC. And lymph node dissection is an efficient supplement to thyroidectomy. Nowadays, prophylactic central neck dissection in PTC is generally accepted while lateral neck dissection is only performed therapeutically (10). According to 2015 ATA Guidelines, prophylactic central neck dissection should be considered in those PTC patients with clinically uninvolved central neck lymph nodes who have advanced primary tumors (T3 or T4) or clinically involved lateral neck nodes. However, lateral neck dissection was suggested to be performed for patients with biopsy-proven metastatic lateral lymphadenopathy. Thus, it’s more difficult to detect lateral compartment metastasis than central compartment metastasis. And if we can determine the predictive factors of lateral compartment metastasis in PTC, it will help us better evaluate the status of the lateral lymph node preoperatively.
According to the new World Health Organization (WHO) classification of thyroid tumors, a PTC less than or equal to 10 mm in diameter is called papillary thyroid microcarcinoma (PTMC) and PTC with a maximum diameter of more than 10 mm is defined as conventional PTC (CPTC) (11). Research has shown that CPTC has a significantly higher LNM rate than PTMC (6,12,13). It may indicate that CPTC has different biological behavior compared with PTMC. Therefore, it’s necessary to separate CPTC patients from PTMC patients. However, only a few studies have explored the clinicopathological characteristics of CPTC with lateral lymph node metastasis (LLNM). We thus conducted a retrospective study to investigate the predictive factors of LLNM in CPTC patients. Compared with previous studies of PTC, the novelty of our study was that we specially focused on analyzing the specific subgroup of PTC, which was called CPTC.
In addition, many studies have shown a connection of the BRAF V600E mutation with invasive pathological feature of PTC, such as LNM, extrathyroidal invasion and even the increased mortality of PTC. BRAF is a serine/threonine kinase which transduces signals downstream of RAS via the mitogen-activated protein kinase (MAPK) pathway (14). BRAF mutation is among the most common genetic change in tumors, particularly in thyroid carcinoma, about 45% cases of PTC occurs this kind of change. The T1799A nucleotide transversion in the BRAF gene (NM_004333) is a prominent oncogenic mutation in PTC. This mutation causes a valine-to-glutamic acid change in codon 600 of the BRAF protein, resulting in BRAF V600E (15). As a significance factor of predicting tumor’s aggressiveness, in consequence, we also investigate the effect of BRAF V600E mutation on CPTC lateral neck metastasis.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-482).
Methods
General clinical materials
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (No. GDREC2020097H). Because of the retrospective nature of the research, the requirement for informed consent was waived.
A total of 681 patients, suspicious for CPTC and admitted to the Department of General Surgery at Guangdong Provincial People’s Hospital between October 2015 and June 2019 were retrospectively reviewed. Preoperative evaluation of lymph nodes included ultrasonic examination (US), neck computed tomography scan (CT), and positron emission tomography-computed tomography (PET-CT). All patients required a US to routinely assess the status of primary tumor and lymph nodes, while CT and PET-CT were used only in some patients as needed. Fine-needle aspiration (FNA) was not routinely used for the diagnosis of lymph node metastasis in our hospital. Total thyroidectomy with bilateral central lymph node dissection (CLND) was performed in all patients. Meanwhile, ipsilateral lateral lymph node dissection (LLND) was performed in those with clinically positive LLNM as detected by preoperative imaging examinations or FNA, or highly suspicious of lateral metastasis intraoperatively. The CLND in our department is performed superiorly to the thyroid cartilage, inferiorly to the innominate vein, externally to the medial carotid sheaths, posteriorly to the prevertebral fascia, and anteriorly to the superficial layer of deep cervical fascia. Meanwhile, the pretracheal and prelaryngeal lymph nodes are also considered a part of the central compartment. LLND is performed superiorly to the posterior belly of digastric muscle, inferiorly to the subclavian vein, externally to the anterior border of the trapezius muscle, and posteriorly to the prevertebral fascia, which is also called level II–V dissection. The pathological diagnosis of surgical specimens was confirmed by at least three pathologists who were experienced in the pathology department of our hospital. For patients who did not receive LLND, if LLNM was discovered by US and confirmed by FNA or surgery within 6 months after initial surgery, they would be considered as having occult LLNM at the time of operation. Those in whom no evidence was found of LLNM by imaging tests within 6 months after initial surgery were considered to not have LLNM.
The expression of BRAF V600E mutant protein in these cases was detected by Ventana IHC method (VE1 antibody). Four-micrometer serial sections from formalin fixed paraffin embedded (FFPE) tissues were cut and placed in a 60 °C constant temperature thermotank overnight. We used a commercially available Ventana MMR IHC Panel (Ventana Medical Systems, Inc. Tucson, Arizona, USA) which includes: anti-BRAF V600E (VE1) Mouse Monoclonal Primary Antibody, ultra-View universal DAB Detection Kit and OptiView Amplication Kit. IHC staining was performed on an Automated Staining System (BenchMark XT, Ventana Medical Systems, Inc. Arizona, USA) and the staining procedures refer to the manufacturer’s kit instructions. Dewaxing time was 2 h, heat pretreatment was 56 min, and antibody was added and then incubated at 37 °C for 36 min, Harris hematoxylin was counterstained for 2 min. The staining results were observed by the microscope and analyzed by two pathologists according to the scoring criteria.
The following information was collected to establish our retrospective database: gender, age, maximum tumor diameter, condition of capsule invasion, condition of extracapsular extension, primary tumor location, number of central lymph nodes (CLNs), condition of diagnosing autoimmune thyroid disease, condition of bilaterality, condition of multifocality, and condition of BRAF V600E mutation.
Some patients were excluded from our study basing on any one of the following criteria: presence of other types or combined with other types of thyroid malignancy, coexistence of other head or neck malignancies, without US examination 6 months after surgery, prior surgery or radiotherapy of the neck, and other distant metastases. Ultimately, a total of 652 patients were eligible for our study.
Statistical analysis
Data collection was performed using Microsoft Excel. Statistical analysis was performed using IBM SPSS statistics 25.0 software. Continuous variables are presented as means with standard deviations. Categorical variables are presented as numbers. Univariate analyses by the χ2 test or Fisher’s exact test was performed to explore the relationships between CPTC and clinicopathologic characteristics. Multivariate analysis was performed by binary logistic regression to investigate the independent factors of LLNM in CPTC. P values <0.05 were considered statistically significant.
Results
Characteristics of patients and tumors
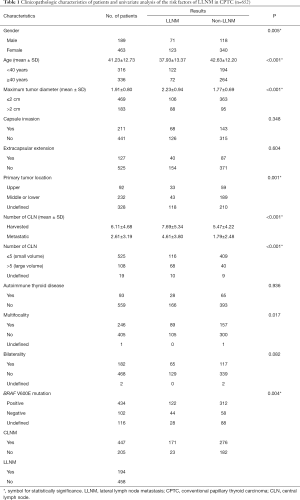
As shown in Table 1, 652 patients who met our criteria were enrolled in this study. There were 189 (29.99%) men and 463 (70.01%) women. Patients were divided into two groups using age of 40 years as the cutoff point, with 316 (48.47%) patients being aged <40 years and 336 (51.53%) being aged ≥40 years. The mean age of was 41.23±12.73 years. Maximum tumor diameter >2 cm was found in 183 (28.07%) patients while 469 (71.93%) tumors were ≤2 cm in diameter. The mean maximum tumor diameter was 1.91±0.80 cm. A total of 211 tumors presented capsule invasion, and 127 tumors presented extracapsular extension. In addition, 92 of the primary tumors were located in the upper lobe, while 232 of the primary tumors were located in the middle or lower lobe. We failed to define the location of 328 tumors because some of them were so massive in size that they occupied the entire lobe of the thyroid. Number of harvested and metastatic CLNs were 6.11±4.68 and 2.61±3.19 respectively. Moreover, 108 patients were classed into the large volume LNM group (more than 5 pathologically confirmed lymph nodes with all involved lymph nodes less than 3 cm in largest dimension) and 525 patients were classed into the small volume LNM group (no more than 5 pathologically confirmed lymph nodes with all involved lymph nodes less than 3 cm in the largest dimension or no central compartment metastasis). Multifocal tumors were found in 246 patients, bilateral tumors were found in 182 patients, and 93 patients were diagnosed with autoimmune thyroid disease pathologically. BRAF V600E mutation-positive was found in 434 (66.56%) patients while 102 (15.64%) were negative. Among all the patients, 194 were diagnosed as LLNM.
Full table
Predictive factors of LLNM in CPTC
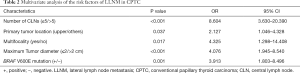
As shown in Table 1, univariate analyses revealed that LLNM in CPTC was significantly related to gender (P=0.005), age (P<0.001), maximum tumor diameter (P<0.001), primary tumor location (P=0.001), multifocality (P=0.017), BRAF V600E mutation (P=0.004), and the number of CLNs (P<0.001). There were no significant differences in other clinicopathological factors such as capsule invasion (P=0.348), extracapsular extension (P=0.604), autoimmune thyroid disease (P=0.936), or bilaterality (P=0.082). The multivariate analysis results are shown in Table 2. A binary logistic regression revealed that multifocality (P=0.017, OR =4.325, 95% CI: 1.298–14.408), maximum tumor diameter (P<0.001, OR =4.076, 95% CI: 1.945–8.540), primary tumor location (P=0.037, OR =2.127, 95% CI: 1.046–4.328), number of CLNM (P<0.001, OR =8.604, 95% CI: 3.630–20.390) and BRAF V600E mutation (P=0.001, OR =3.913, 95% CI: 1.803–8.496) were independent predictors of LLNM in CPTC.
Full table
Discussion
We analyzed 652 patients with pathologically diagnosed CPTC and found that the incidence rate of LLNM was 29.75% (194 of 652 patients). In our study, we found that a large volume of CLNM was an independent risk factor of LLNM (P<0.001, OR =8.604, 95% CI: 3.630–20.390). CLNM was also confirmed to be a predictive factor of LLNM in PTC patients by other researchers (16-18). Ducoudray et al. demonstrated that the incidence of LLNM in patients with 3 or more metastatic CLNs was significantly higher than those without CLNM (P<0.0001, OR =6.416) (1,2,19). According to the 2015 American Thyroid Association (ATA) Risk Stratification System, patients with more than 5 pathologically confirmed lymph nodes with the largest dimension less than 3 cm were classified as intermediate risk of recurrence, and those with any lymph nodes equal or larger than 3 cm in largest dimension were classified as high risk (10). This shows the relationship between large volume LNM and recurrence risk, while our study found a relation between large volume LNM with the and the risk of LLNM in CPTC. Moreover, a large-scale study comprising 69,757 patients reported that as the number of metastatic cervical lymph nodes increased (up to 6 lymph nodes) survival decreased (3).
With univariate and multivariate analyses, we found tumors located in the upper lobe showed a significantly higher rate of LLNM than those located in the other side of the lobe (P=0.037, OR =2.127, 95% CI: 1.046–4.328). This finding was consistent with the previous studies of PTMC (20,21), and higher tumor burden might be one of the explanations for this. Compared to the other sides, the upper lobe has a relatively small amount of tissue, due to the butterfly shape of the thyroid gland. Consequently, same side of the tumor in the upper lobe has a higher tumor burden than that in the middle and lower lobe (22). Another explanation is that the tumor located in the upper lobe is able to spread directly to the ipsilateral lateral lymph compartment via lymphatic ducts along the superior venous vessels (8,23,24). Through these channels, tumors can metastasize directly to the lateral compartment by bypassing the central compartment, which is defined as skip metastasis. Our study found skip metastasis in in 23 tumors.
Multifocality of tumor was also a risk factor for LLNM in CPTC (P=0.017, OR =4.325, 95% CI: 1.298–14.408). Previous studies have already correlated multifocality with regional LNM (25,26). It was believed that the clonal origin of multifocality is caused by the intraglandular dissemination of a single primary tumor, which indicates increased aggressiveness (27). However, Nam et al. thought that multiple foci might arise as distinct cancers and have individual characteristics of their own (7). In a study of 60 patients, it was found that at least 30% of multifocal PTCs developed through distinct molecular alterations (28). Thus, the increased number of tumors, rather than the aggressiveness of the primary tumor, might result in the increased possibility of LNM.
CPTC has been proven to exhibit a significantly higher rate in LLNM than PTMC (13,29). Our study used 2 cm as the cutoff point of tumor diameter, according to the American Joint Committee on Cancer (AJCC) 8th edition of tumor-node-metastasis (TNM) classification system, and found tumor diameter ≥2 cm was a predictive factor for LLNM in CPTC patients (P<0.001, OR =4.076, 95% CI: 1.945–8.540). Another finding was that BRAF V600E mutation-negative was more likely to present with LLNM than mutation-positive (P=0.001, OR =3.913, 95% CI: 1.803–8.496). This was unexpected because previous reports demonstrated the association of BRAF V600E mutation with aggressive clinicopathologic characteristics in PTC (30-32). In addition, univariate analysis revealed male and younger (age <40 years) patients were statistically risk factors, although multivariate analysis found no significant difference.
Our study aimed to identify predictive factors of LLNM in CPTC in order to help surgeons build a strategy when facing tumors with a maximum diameter of more than 10 mm. However, there were several limitations in our study. Firstly, our study was only a single-center retrospective analysis. Also, selection bias was inevitable because prophylactic lateral compartment dissection was not recommended by international guidelines, and so all cases enrolled were already confirmed with LLNM. Therefore, some of our findings need to be further explored by multicenter prospective studies.
Conclusions
In our study, LLNM of CPTC was significantly associated with gender, age, number of CLN, primary tumor location, multifocality, maximum tumor diameter, and BRAF V600E mutation. The independent predictors of LLNM in CPTC were large volume of CLNM, tumor located in the upper lobe, multifocality, tumor diameter >2 cm, and BRAF V600E mutation-negative. These risk factors could help us devise individualized therapy strategies when facing patients suspicious of LLNM. However, larger multicenter studies still need to be conducted to further confirm our study of LLNM in CPTC.
Acknowledgments
Funding: This work was supported by grants from the Natural Science Foundation from Guangdong Province (No. 2020A1515010127), Scientific Research Staring Foundation for the Returned Overseas from Guangdong Provincial People’s Hospital (No. 2017x02) and Guangdong Province Science Fund for Outstanding Young Medical Scholars (KJ012019441).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-482
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-482
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-482). The authors report grants from The Natural Science Foundation from Guangdong Province, grants from Scientific Research Staring Foundation for the Returned Overseas from Guangdong Provincial People’s Hospital, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (No. GDREC2020097H). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Vaccarella S, Dal Maso L, Laversanne M, et al. The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid 2015;25:1127-36. [Crossref] [PubMed]
- Adam MA, Pura J, Goffredo P, et al. Presence and Number of Lymph Node Metastases Are Associated With Compromised Survival for Patients Younger Than Age 45 Years With Papillary Thyroid Cancer. J Clin Oncol 2015;33:2370-5. [Crossref] [PubMed]
- Lira RB, Chulam TC, Kowalski LP. Variations and results of retroauricular robotic thyroid surgery associated or not with neck dissection. Gland Surg 2018;7:S42-52. [Crossref] [PubMed]
- Kim H, Kim TH, Choe JH, et al. Patterns of Initial Recurrence in Completely Resected Papillary Thyroid Carcinoma. Thyroid 2017;27:908-14. [Crossref] [PubMed]
- Zhao H, Huang T, Li H. Risk factors for skip metastasis and lateral lymph node metastasis of papillary thyroid cancer. Surgery 2019;166:55-60. [Crossref] [PubMed]
- Liu Z, Chen S, Huang Y, et al. Synergic effects of histology subtype, tumor size, and lymph node metastasis on distant metastasis in differentiated thyroid cancer. Ann Transl Med 2019;7:533. [Crossref] [PubMed]
- Likhterov I, Reis LL, Urken ML. Central compartment management in patients with papillary thyroid cancer presenting with metastatic disease to the lateral neck: Anatomic pathways of lymphatic spread. Head Neck 2017;39:853-9. [Crossref] [PubMed]
- Attard A, Paladino NC, Lo Monte AI, et al. Skip metastases to lateral cervical lymph nodes in differentiated thyroid cancer: a systematic review. BMC Surg 2019;18:112. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Kakudo K, Bychkov A, Bai Y, et al. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol Int 2018;68:641-64.
- Liu X, Zhu L, Wang Z, et al. Evolutionary features of thyroid cancer in patients with thyroidectomies from 2008 to 2013 in China. Sci Rep 2016;6:28414. [Crossref] [PubMed]
- So YK, Kim MJ, Kim S, et al. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg 2018;50:94-103. [Crossref] [PubMed]
- Giordano M, Zaed I. Value of magnetic resonance imaging in predicting BRAF mutation in craniopharyngiomas. Transl Cancer Res 2019;8:S97-8. [Crossref]
- Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013;309:1493-501. [Crossref] [PubMed]
- Zhang L, Wei WJ, Ji QH, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab 2012;97:1250-7. [Crossref] [PubMed]
- Kim JW, Roh JL, Gong G, et al. Extent of Extrathyroidal Extension as a Significant Predictor of Nodal Metastasis and Extranodal Extension in Patients with Papillary Thyroid Carcinoma. Ann Surg Oncol 2017;24:460-8. [Crossref] [PubMed]
- Kim SK, Park I, Woo JW, et al. Predictive Factors for Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Ann Surg Oncol 2016;23:2866-73. [Crossref] [PubMed]
- Ducoudray R, Tresallet C, Godiris-Petit G, et al. Prophylactic lymph node dissection in papillary thyroid carcinoma: is there a place for lateral neck dissection? World J Surg 2013;37:1584-91. [Crossref] [PubMed]
- Jeon MJ, Chung MS, Kwon H, et al. Features of papillary thyroid microcarcinoma associated with lateral cervical lymph node metastasis. Clin Endocrinol (Oxf) 2017;86:845-51. [Crossref] [PubMed]
- Luo Y, Zhao Y, Chen K, et al. Clinical analysis of cervical lymph node metastasis risk factors in patients with papillary thyroid microcarcinoma. J Endocrinol Invest 2019;42:227-36. [Crossref] [PubMed]
- Back K, Kim JS, Kim JH, et al. Superior Located Papillary Thyroid Microcarcinoma is a Risk Factor for Lateral Lymph Node Metastasis. Ann Surg Oncol 2019;26:3992-4001. [Crossref] [PubMed]
- Ito Y, Tomoda C, Uruno T, et al. Papillary microcarcinoma of the thyroid: how should it be treated? World J Surg 2004;28:1115-21. [Crossref] [PubMed]
- Dralle H, Machens A. Surgical management of the lateral neck compartment for metastatic thyroid cancer. Curr Opin Oncol 2013;25:20-6. [Crossref] [PubMed]
- Tam AA, Ozdemir D, Cuhaci N, et al. Association of multifocality, tumor number, and total tumor diameter with clinicopathological features in papillary thyroid cancer. Endocrine 2016;53:774-83. [Crossref] [PubMed]
- Kuo SF, Lin SF, Chao TC, et al. Prognosis of multifocal papillary thyroid carcinoma. Int J Endocrinol 2013;2013:809382. [Crossref] [PubMed]
- Wang W, Wang H, Teng X, et al. Clonal analysis of bilateral, recurrent, and metastatic papillary thyroid carcinomas. Hum Pathol 2010;41:1299-309. [Crossref] [PubMed]
- Bansal M, Gandhi M, Ferris LR, et al. Molecular and histopathologic characteristics of multifocal papillary thyroid carcinoma. Am J Surg Pathol 2013;37:1586-91. [Crossref] [PubMed]
- Feng JW, Yang XH, Wu BQ, et al. Predictive factors for central lymph node and lateral cervical lymph node metastases in papillary thyroid carcinoma. Clin Transl Oncol 2019;21:1482-91. [Crossref] [PubMed]
- Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 2015;33:42-50. [Crossref] [PubMed]
- Han PA, Kim HS, Cho S, et al. Association of BRAF V600E Mutation and MicroRNA Expression with Central Lymph Node Metastases in Papillary Thyroid Cancer: A Prospective Study from Four Endocrine Surgery Centers. Thyroid 2016;26:532-42. [Crossref] [PubMed]
- Zhang Q, Liu SZ, Zhang Q, et al. Meta-Analyses of Association Between BRAF(V600E) Mutation and Clinicopathological Features of Papillary Thyroid Carcinoma. Cell Physiol Biochem 2016;38:763-76. [Crossref] [PubMed]


