
Inflammatory myofibroblastic tumor of the neck with thyroid invasion: a case report and literature review
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare disease, first reported in 1939 in the lung or pleura (1). In the past, most scholars believed that inflammatory pseudotumor were benign tumor-like lesions; however, in recent years, some inflammatory pseudotumor have been identified as having malignant potential, including for recurrence, infiltration, and metastasis. According to the World Health Organization (WHO) (2) classified IMTS as a clear, low-grade malignant tumor. The latest WHO soft tissue tumor classification expert group has classified IMT as a low-grade malignant, or borderline, mesenchymal tumor (3). The incidence of IMT in the head and neck accounts for about 5% of total cases (4,5). Head and neck IMT can occur in the orbit, throat, mouth, tonsil, parapharyngeal space, thyroid gland, parotid gland, and lacrimal gland, among other sites (4). A slight male preponderance (M: F ratio, 1.3) was reported in previous studies (6). It is rare for neck IMT to involve the thyroid and esophagus simultaneously. In this paper, we report a single case of neck IMT with invasion of the thyroid and esophagus.
We present the following article in accordance with the CARE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-355.
Case presentation
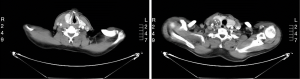
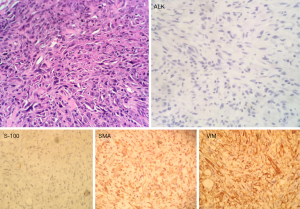
A male patient of 66 years old was admitted to the hospital with a primary complaint of a “neck mass with hoarseness for a month”. The patient used to be a cook, with good health and without family history, who hadn’t received radiation or other treatment before. A tough mass of 4×3 cm could be felt in the right thyroid, which moved when the patient swallowed. Below this mass was another growth of approximately 6×4 cm, which was hard with an unambiguous boundary and not mobile. Enhanced computed tomography (CT) examination of the thyroid showed that the right lobe contained a mixed-density mass, protruding outside the thyroid, while inside the thyroid, a slightly calcified area with an unclear boundary was visible. The radial line of the shaft was approximately 3.7–2.6 cm, and the right common carotid artery was obstructed. The laryngeal cavity was narrowed with no destruction of the laryngeal cartilage (Figure 1). Preoperative diagnosis: Malignant tumor of neck with tendency to thyroid origin. Surgery was conducted on Nov. 15, 2018. The neck mass was hard and fixed with unclear boundaries, and located in the posterior part of the right thyroid gland. Further exploration revealed that the tumor was approximately 10×8 cm, and surrounded the internal jugular vein and common carotid artery. The upper part of the tumor reached the larynx, with the lower boundary at the supraclavicular, lateral margins reaching to the outer edge of the sternocleidomastoid muscle, and the posterior to the anterior vertebra. The medial part of the tumor was closely associated with the right lobe of the thyroid gland and esophagus. Resection of the right neck lymph nodes (groups 2, 3, and 4), right thyroidectomy, and left subtotal thyroidectomy were conducted. Routine pathological examination following surgery demonstrated that the gray matter was solid, without a capsule or small focal ischemic necrotic area. The tumor dimensions were 8×6×2 cm. Microscopic examination revealed fusiform cells closely arranged in banded bundles, with infiltration of plasmacytes and lymphocytes. Together with immunohistochemical staining, these observations allowed diagnosis of the tumor as cervical fusiform cell cancer, with chronic inflammatory cell infiltration, which was classified as an IMT. Invasive tumor growth involved the thyroid and caused rhabdomyolysis of tissues and lymph nodes without metastasis. Immuno-histochemistry results (Figure 2) were as follows: ALK (−), TG (−), CK (1), TG (−), TTF1 (−), MC (−), Vim (+++), LCA (−), CD31 (+), CD34 (−), Fli-1 (−), s-100 (−), SMA (+), Desmin (−), and ki67 (30%+). No adjuvant therapy was administered after surgery (patient rejected) and there was no tumor recurrence or metastasis detected at 6-month follow-up. The patient’s family has signed the written consent.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Etiology of IMT
The exact etiology and pathogenesis of IMT are yet to be clarified. Virus-induced trauma, surgery, autoimmune etiology, inflammation, infection, and abnormal responses to long-standing exogenous stimuli in the body, dominated by myofibroblast proliferation, can lead to IMT development (7,8). The patient described here had no history of alcohol consumption, smoking, injury, or other diseases.
Abnormal expression of the anaplastic lymphoma kinase (ALK) gene and protein has been reported in IMTs. Although some IMTs are ALK-negative, these are characterized by other genetic abnormalities, such as the TFG-ROS1 fusion gene. Detection of rearrangements of the ETV6 gene and the ETV6-NTRK3 fusion gene confirmed that IMT was true tumor, rather than an inflammatory pseudotumor (9,10). ALK is a receptor tyrosine kinase and abnormal expression of the NPM-ALK fusion protein was first detected in anaplastic large cell lymphoma (ALCL) by Morris et al., while abnormal expression of ALK has also been reported in IMT and non-small cell lung cancer (NSCLC) (11). Genes fused with ALK in IMT reported to date include TPM3, TPM4, CLTC, and CARS. Abnormal expression of the ALK gene can lead to aberrant ALK protein expression, which induce abnormal proliferation of myo-fibroblastic cells and thereby promote tumorigenesis (12).
Clinical and imaging findings in IMT
The clinical manifestations of IMT are determined by the site of origin and the effects of the mass (13). Lung IMT can cause chest pain and dyspnea (14), while patients with abdominal disease can exhibit jaundice and gastrointestinal obstruction (15). The clinical manifestations associated with head and neck IMT resemble inflammation. Patients often present with progressive swelling of the maxillofacial region, with local pain as the main symptom. According to disease location, nasal cavity IMT can present as nasal congestion, nosebleed, and olfactory loss, tonsil and tongue IMT in the oropharynx can cause dysphagia and orbital IMT may result in epiphora, eye distension, and exophthalmos (16). Local swelling and hoarseness were the primary symptoms in the case reported here.
The imaging features of IMT also lack specificity, and IMT cannot be distinguished from inflammation by either CT or MRI. Differential diagnoses include malignant tumors, such as lymphomas, rhabdomyosarcomas, and teratomas. On CT, IMT mainly manifests as soft tissue mass, with variable degrees of enhancement, uneven density and few clearly enhanced necrotic or calcified regions (17). In contrast, MRI can better reflect inner IMT tumor tissue and reveal the relationship between the lesion and important surrounding tissue structures. Most lesions are soft tissue masses with unclear margins and irregular shape. There are some reports of invasion of adjacent muscles or destruction of bone (16). In the absence of pathognomonic features on imaging, diagnosis is usually only achieved by histopathologic examination of tumor specimens.
Pathological manifestations of IMT
Pathology is the gold standard for IMT diagnosis. Some IMT have clear margins and a false envelope, while others, particularly recurrent tumors, have irregular boundaries. In recurrent tumors, the tumor body is irregular within unclear margins and invasion of the surrounding muscle or bone tissue. These tumors adhere to surrounding tissues and grow in an infiltrating manner (16).
According to the distribution of fibroblasts/myofibroblasts, inflammatory cells, and interstitial characteristics, IMT can be divided into three basic histologic configurations: (I) a myxoid-vascular pattern, resembling nodular fasciitis; (II) a compact spindle cell pattern, resembling a variety of spindle cell neoplasms; and (III) a hypocellular fibrous pattern, resembling a scar or desmoid fibromatosis (16).
The pathological findings in our case included fusiform cells accompanied by inflammatory cell infiltration, consistent with the second histological type. ALK expression has recently been proposed as a good marker for IMT, with 50% to 70% of IMT samples ALK-positive (16,17). Immunohistochemical staining shows diffuse positivity for vimentin (Vim), while staining with the myogenic antibodies, smooth muscle actin (SMA) and muscle-specific actin (MSA), is localized or diffusely positive, and Desmin, Actin, Calponin, and ALK-1 are usually positive. S100, Myglobin, CD34, CD117, CD21, CD23, and Caldesmon are often negative. Immunohistochemical staining is important for diagnosis and differential diagnosis. Positive expression of SAM, MSA, and VIM support diagnosis of IMT, which is morphologically characterized by fibroblasts and smooth muscle cells, allowing these tumors to be distinguished from those comprising cells of fibroblast and smooth muscle cell origin (18).
In approximately 50% to 60% of cases, ALK protein can be detected, and there is a good correlation with ALK gene rearrangement. Different fusion genes result in varying staining patterns (19). The ALK gene was negative in our case.
Treatment of IMT
IMT is generally considered a benign tumor, for which the primary therapeutic approach is surgery, if the anatomic location permits resection; however, for patients with tumors not amenable to surgery, treatment generally involves combined therapies. Nevertheless, chemotherapy, radiotherapy, immunomodulation with corticosteroids, and non-steroidal anti-inflammatory drugs have been reported not to be effective against individual invasive tumors (20). There is no consensus on effective treatment for head and neck IMT. At present, commonly used treatment approaches include surgery and conservative treatment (Medication), but primarily surgery (5). The operative principle for head and neck IMT is that radical resection should be performed with the aim of preserving important structures as far as possible, prioritizing function. For patients where tumors are incompletely resected, or where the resection margins are positive, postoperative radiotherapy and chemotherapy is required (16).
Positive ALK expression often indicates poor prognosis; however, ALK-targeted drug therapy for ALK-positive IMT is promising. In recent years, ALK-I expression rates in IMT have been identified as high, with positive rates by immunohistochemistry up to 89%. ALK can also activate other tyrosine kinases, which are associated with local tumor recurrence and metastasis (21). Abnormal ALK expression and rearrangement of the ALK gene have been proposed as valuable for histopathological diagnosis and differential diagnosis in IMT. ALK detection in IMT tissue samples may also be helpful for directing targeted therapy, as ALK inhibitors can be used to treat tumors positive for this protein (22). Crizotinib is a tyrosine kinase inhibitor of the ALK receptor, which blocks activity of ALK fusion proteins by competing with adenosine triphosphate for binding to the ALK receptor, thereby blocking the transmission of pathological signals and inhibiting tumor cell expansion (23). Crizotinib has been approved by FDA for the treatment of ALK-positive NSCLC and clinical trials have confirmed that Crizotinib is also effective for ALK-positive ALCL and neuroblastoma (24). Moreover, there have been case reports showing that treatment of some cases of ALK-positive IMT with Crizotinib, particularly those where surgery is difficult or there is postoperative recurrence, could effectively reduce tumor size and inhibit tumor growth (25).
Prognosis of IMT
The local recurrence rate of extrapulmonary IMT is approximately 25%, which is related to the anatomic location, resectability, and whether the tumor is solitary or multi-nodular. For head and neck IMT, if surgical resection is incomplete, recurrence rates can be as high as 50% (16), while the metastasis rate is less than 2%. This may be associated with specific fusion genes, such as ALK-RANBP2 (19), and metastasis is more common in ALK-negative IMT (24). Prognostication of the possibility of recurrence and metastasis after surgical resection requires clinical, biological, and imaging follow-up (25).
In summary, this report has two strengths: (I) IMT is a low-incidence, borderline, or low-grade mesenchymal tumor, with few malignant features, some of which indicate the potential for recurrence, infiltration, and metastasis. Clear diagnosis primarily depends on pathological and immunohistochemical examination. (II) IMT is well characterized at the clinical pathological levels, and associated protein and gene abnormalities have been analyzed, which facilitates both assessment of diagnosis and prognosis, and identification of treatment targets, to the benefit of patients. However, limitations for this case should be noticed: the patient had poor compliance and did not receive further treatment; Only follow-up for half a year, close follow-up is necessary for patients with IMTs after surgical resection.
Acknowledgments
Funding: This study was supported by the Excellent youth project of the Fourth Affiliated Hospital of Harbin Medical University (Grant No. HYDSYYXQN202006, principal investigator Jinlu Zhao), the youth project of Science and technology innovation project of Heilongjiang Academy of traditional Chinese Medicine (Grant No. ZHY19-080, principal investigator Jinlu Zhao).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-355
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-355). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient. The study was approved by Ethics Committee of the Fourth Affiliated Hospital of Harbin Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brunn H. Two interesting benign lung tumors of contradictory histopathology. Thorac Surg 1939;9:119.
- Fletcher CDM, Unni KK, Mertens F. World Health Organization classification of tumors. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press, 2002:48-106.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classification of Tumours of Soft Tissue and bone. Lyon: IARC Press, 2013:83-4.
- Al-Humidi A, Al-Khamiss A. Inflammatory myofibroblastic tumor arising in the external ear: unexpected location (case report). Int J Health Sci (Qassim) 2015;9:201-5. [Crossref] [PubMed]
- Tao J, Zhou ML, Zhou SH. Inflammatory myofibroblastic tumors of the head and neck. Int J Clin Exp Med 2015;8:1604-10. [PubMed]
- Mehta B, Mascarenhas L, Zhou S. Inflammatory myofibroblastic tumors in childhood. Pediatr Hematol Oncol 2013;30:640-5. [Crossref] [PubMed]
- Kovach SJ, Fischer AC, Katzman PJ, et al. Inflammatory myofibroblastic tumors. J Surg Oncol 2006;94:385-91. [Crossref] [PubMed]
- Tao YL, Wang ZJ, Han JG, et al. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: a case report. World J Gastroenterol 2012;18:7100-3. [Crossref] [PubMed]
- Coffin CM, Patel A, Perkins S, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol 2001;14:569-76. [Crossref] [PubMed]
- Yamamoto H, Yoshida A, Taguchi K, et al. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology 2016;69:72-83. [Crossref] [PubMed]
- Morris SW, Kirstein MN, Valentine MB. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994;263:1281-4. [Crossref] [PubMed]
- Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 2000;157:377-84. [Crossref] [PubMed]
- Karnak I, Senocak ME, Ciftci AO, et al. Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg 2001;36:908-12. [Crossref] [PubMed]
- Pettinato G, Manivel JC, De Rosa N, et al. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immune-histochemical and ultrastructural observations. Am J Clin Pathol 1990;94:538-46. [Crossref] [PubMed]
- Pisciotto PT, Gray GF Jr, Miller DR. Abdominal plasma cell pseudotumor. J Pediatr 1978;93:628-30. [Crossref] [PubMed]
- Ong HS, Ji T, Zhang CP, et al. Head and neck inflammatory myofibroblastic tumor (IMT): evaluation of clinicopathologic and prognostic features. Oral Oncol 2012;48:141-8. [Crossref] [PubMed]
- Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509-20. [Crossref] [PubMed]
- Ge R, Liu C, Yin X, et al. Clinicopathologic characteristics of inflammatory pseudotumor-like follicular dendritic cell sarcoma. Int J Clin Exp Pathol 2014;7:2421-9. [PubMed]
- Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immune- histochemical study. Am J Surg Pathol 2001;25:1364-71. [Crossref] [PubMed]
- Yang X, Miao R, Yang H, et al. A retrospective and comparative study of inflammatory myofibroblastic tumor of the liver. J Gastroenterol Hepatol 2015;30:885-90. [Crossref] [PubMed]
- Pecoraro Y, Diso D, Anile M, et al. Primary inflammatory myofibroblastic tumor of the trachea. Respirol Case Rep 2014;2:147-9. [Crossref] [PubMed]
- Yi ES, Chung JH, Kulig K, et al. Detection of anaplastic lymphoma kinase (ALK) gene rearrangement in non-small cell lung cancer and related issues in ALK inhibitor therapy: a literature review. Mol Diagn Ther 2012;16:143-50. [Crossref] [PubMed]
- Hallberg B, Palmer RH. Crizotinib-latest champion in the cancer wars? N Engl J Med 2010;363:1760-2. [Crossref] [PubMed]
- Croegaert K, Kolesar JM. Role of anaplastic lymphoma kinase inhibition in the treatment of non-small-cell lung cancer. Am J Health Syst Pharm 2015;72:1456-62. [Crossref] [PubMed]
- Gaudichon J, Jeanne-Pasquier C, Deparis M, et al. Complete and repeated response of a metastatic ALK-rearranged inflammatory myofibroblastic tumor to crizotinib in a teenage girl. J Pediatr Hematol Oncol 2016;38:308-11. [Crossref] [PubMed]


