
Atypia of undetermined significance/follicular lesion of undetermined significance: Asian vs. non-Asian practice, and the Singapore experience
Introduction
It is an undisputed fact that regional differences exist in diagnostic and management practices between different geographic regions. Much of this may be related to epidemiological variations, however, a combination of cultural factors and national and regional healthcare infrastructure does play a role.
In the recent literature, there has been some documentation of the differences in diagnostic thresholds and/or management of thyroid nodules between the East and West (1,2). For example, Bychkov et al. has demonstrated that the impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on Bethesda category outcomes is less marked in Asia than in the West (2,3). The appreciation of regional differences is highly relevant in current global practice because of the recent worldwide movement towards applying international consensus guidelines for cytologic terminology in many different organ systems, with the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) being one of the pioneer consensus systems in non-gynecologic cytology (4).
One of the most challenging Bethesda categories is the atypical category—atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS). Not only is there diversity in cytomorphologic subgroups within this category, some diagnostic criteria may overlap with those of other Bethesda categories. Indeed, a number of independent studies have sought to address the differential risk of malignancy (ROM) associated with the presence of nuclear atypia within this category in both Asian and non-Asian study groups (5-16). Understandably, the AUS/FLUS diagnosis is also heavily influenced by individual and institutional experience, and bears an inherent element of subjectivity in interpretation. This is reflected in the wide variation in both the reported incidence rates and malignancy rates in this category. Recently, Vuong et al. demonstrated regional differences in the resection rates (RRs) and ROM of AUS/FLUS, suggesting that Asian clinicians as a whole tend to adopt a more conservative approach in their management of AUS/FLUS nodules as compared to their Western counterparts (1).
In view of its diagnostic and therapeutic heterogeneity, we draw focus to the AUS/FLUS category, comparing the practice of thyroid cytopathology between Asian and non-Asian countries, with a particular emphasis on the Singapore experience. We aim to characterize the differences between Asian and non-Asian practice in terms of its incidence, RRs, rates of repeat fine needle aspiration (rFNA), and ROMs. In addition, we review the outcomes of repeat FNA and the differential ROMs amongst cytomorphologic subgroups—cases with nuclear atypia vs. architectural atypia only. Where possible, we will also compare the prevalence of well-differentiated neoplasms amongst surgically resected cases.
Lastly, we will comment on the different practices in molecular interrogation of AUS/FLUS nodules between Asian and non-Asian countries. For this comparison, we draw our information from studies that focus on AUS/FLUS outcomes rather than primarily on molecular testing.
Methods and materials
Identification and selection of studies
We searched for relevant articles in PubMed from January 2007 to August 2019 using the search term “(fine needle OR cytology) AND thyroid AND Bethesda”. Study titles and abstracts were screened for candidate articles. Studies were included if they fulfilled the following criteria: (I) reporting of thyroid FNA results using TBSRTC 2009 or 2017, and (II) adequate data for AUS/FLUS cases provided for either of the following parameters of practice:
- Incidence;
- RR, ROM and overall risk of malignancy (OROM);
- Rate of repeat FNA (rFNA) and rate of a more definitive Bethesda category on rFNA;
- RR without rFNA, ROM without rFNA, ROM with rFNA;
- ROM for the AUS/FLUS subgroups of architectural atypia (AUS-A) and nuclear atypia (AUS-N). Any cases with nuclear atypia (regardless of whether architectural atypia was present) were included in the AUS-N cohort. Subgroups with Hürthle cell change and atypical lymphoid cells were not examined.
The exclusion criteria for the ROM analyses were: (I) studies specifying the use of other risk predictors such as molecular or ultrasound findings to select for surgery, (II) studies using non-consecutive cases, (III) studies that used core needle biopsy as an intermediate form of assessment, (IV) studies that used non-surgical follow-up to determine outcomes, (V) case reports, (VI) reviews and meta-analyses.
Full-text screening and data extraction
The full-text of candidate articles was screened and data extracted into a predefined dataset. The following data points from all the included studies were extracted: year of publication, institution, city, country, period of study. For each parameter below, the following data points were extracted:
- Incidence: number of FNAs performed and number of AUS/FLUS cases;
- RR, ROM and OROM: number of AUS/FLUS cases, number of surgical resections and number of malignancies diagnosed on histology. Malignancy inferred from non-surgical outcomes (i.e., core needle biopsy or imaging) were not included. NIFTP and tumors of uncertain malignant potential were included in the calculation of malignancy. Papillary microcarcinomas were only included in the malignant follow-up category if they were specifically correlated with the nodule that underwent FNA. Studies with less than 30 surgical resections were excluded from calculations of RR, ROM and OROM;
- Rate of rFNA and rate of a more definitive Bethesda category on repeat FNA: Number of AUS/FLUS, number of rFNA, and number of rFNA yielding a more definitive Bethesda category. “More definitive Bethesda category” was defined as Bethesda categories with a more definite management pathway, i.e., benign, follicular neoplasm (FN), suspicious for malignancy (SM) or malignant;
- RR without rFNA, ROM without rFNA, ROM with rFNA: number of AUS/FLUS, number of surgical resections without repeat FNA, number of malignancies on resection histology without repeat FNA, number of surgical resections after repeat FNA, and number of malignancies on resection histology after repeat FNA;
- ROM of AUS/FLUS subgroups: number of AUS-A and AUS-N, number of surgical resections of AUS-A and AUS-N, and number of malignancies on resection in AUS-A and AUS-N. Where reported, the incidence of the two cytomorphological subgroups and the final histologic diagnoses were noted. Studies with less than 30 surgical resections were excluded from calculations of ROM.
For studies that contained potentially overlapping cases from the same institution, the study with the larger number of FNAs was selected. For studies that compared pre- and post-Bethesda prevalence, only cases that were classified post-Bethesda were included. For studies with cases from both Asian and non-Asian institutions, the cases were extracted and analyzed separately according to the geographical region.
Additionally, studies were also screened for molecular testing and specific histologic outcomes. The following data were reviewed where available: (I) type of molecular tests performed and their results, (II) in studies with at least 50 surgically resected cases, the proportion of papillary thyroid carcinoma (PTC), follicular variant PTC (FVPTC), and FNs, including follicular adenoma (FA) and follicular carcinoma (FC), among surgically resected cases.
Data analysis
The following definitions were applied: incidence of AUS/FLUS is equivalent to the proportion of AUS/FLUS cases among total number of FNAs performed. The RR is equivalent to the proportion of cases in a specified group that underwent surgical resection. The ROM is equivalent to the proportion of malignant cases confirmed by histopathological examination among the surgically resected cases of a specified group. The OROM is equivalent to the proportion of malignant cases confirmed by histopathological examination among all the cases in a specified group (both with and without resection). The rate of rFNA is equivalent to the proportion of AUS/FLUS cases that received one or more repeat FNAs.
Statistical analysis was performed using the statistical software JAMOVI (https://www.jamovi.org), which is built in the R statistical language. Each parameter was independently evaluated with meta-analysis of proportion using DeSimonian-Laird method and 95% CIs were pooled using a random-effect model. The comparison of proportions between Asian and Non-Asian studies were performed using subgroup analysis.
Assessment of publication or small study bias
To assess the presence of publication bias and small study bias, funnel plots of effect estimates from individual studies were performed. Funnel plot asymmetry was determined using the rank correlation test and regression test. A P value of <0.05 was considered statistically significant for the presence of funnel plot asymmetry, which indicates the presence of publication or small study bias.
Results
We identified 859 papers for title and abstract screening and included 210 for full text review. One hundred and twenty studies were eventually selected for extraction of data. Asian series included those from South-East Asia (Singapore and Thailand), East Asia (mainland China, Taiwan, Japan and Korea), South Asia (India and Bangladesh), and West Asia (Kingdom of Saudi Arabia, United Arab Emirates, Turkey, Israel, and Egypt). Non-Asian series included those from the United States of America (USA), Europe (France, Poland, Spain, Finland, Macedonia and Czech Republic), Canada, Brazil, South Africa and Australia.
Incidence of AUS/FLUS
Forty-six Asian series and 43 non-Asian series were included in the pooled analysis of AUS/FLUS incidence (Table 1). The total number of cases amongst the Asian and non-Asian cohorts were 260,169 and 149,460 in this analysis. The incidence of the diagnosis of AUS/FLUS was not found to be significantly different between the Asian and non-Asian cohorts [8.8% (95% CI, 7.4–10.2%) vs. 9.1% (95% CI, 7.9–10.3%), P=0.69]. The incidence of AUS/FLUS in a single tertiary referral center in Singapore was 6.4% (16).
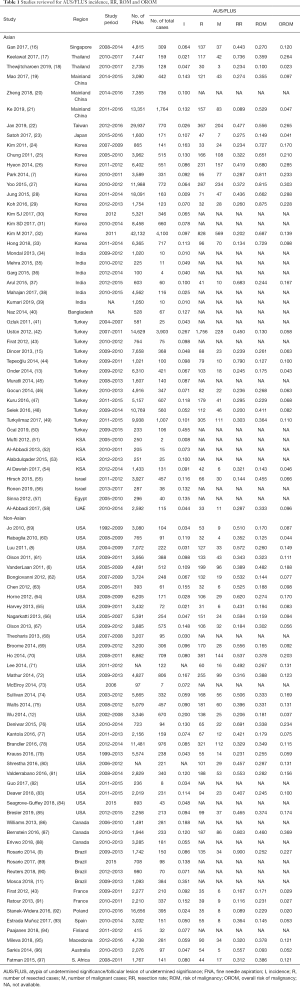
Full table
RR, ROM and OROM within AUS/FLUS
Twenty-eight Asian series and 35 non-Asian series were included in the pooled analysis of RR, ROM and OROM (Table 1). This yielded a total of 19,232 cases in the Asian studies and 10,567 in the non-Asian studies. There were statistically significant differences in both the RRs and ROMs. The RR in the Asian cohort was significantly lower than that of the non-Asian cohort [33.2% (95% CI, 27.7–38.8%) vs. 43.4% (95% CI, 36.7–50.2%), P=0.02] (Figure 1A,B). Conversely, the ROM in the Asian cohort was higher than that of the non-Asian cohort [43.2% (95% CI, 32.4–54.0%) vs. 26.8% (95% CI, 23.3–30.3%), P=0.005] (Figure 2A,B).


The OROM was not significantly different between the Asian and non-Asian cohorts [13.0% (95% CI, 10.6–15.4%) vs. 11.4% (95% CI, 9.2–13.7%), P=0.32].
In Singapore, the RR in a single institution was 44.3%, the ROM was 27.0% and the OROM was 12.0%.
Repeat FNA in AUS/FLUS and outcomes
Nine Asian and 16 non-Asian series were included in the pooled analysis of the rate of rFNAs after an initial FNA diagnosis of AUS/FLUS, and the outcomes of the rFNA (Table 2). In total, there were 7,541 cases amongst the Asian studies and 4,479 cases in the non-Asian studies. There were no regional differences in the rate of rFNA [Asian, 29.4% (95% CI, 22.7–36.2%); non-Asian, 28.0% (95% CI, 22.5–33.5%), P=0.74] or the rate of a more definitive Bethesda category on rFNA [Asian, 65.9% (95% CI, 49.3–82.5%); non-Asian, 61.2% (95% CI, 55.6–66.7%), P=0.62].
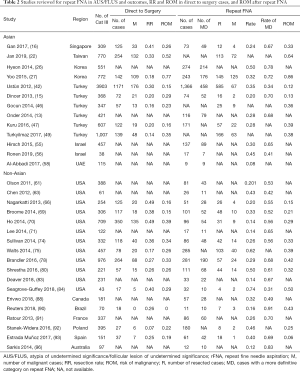
Full table
In the Singapore series, the rate of rFNA was 23.6%, whilst the rate of a more definitive Bethesda category on rFNA was 67.1%.
RR and ROM for direct to surgery cases; ROM after repeat FNA
Eight Asian and 11 non-Asian series were included in the pooled analysis of the RR and ROM without repeat FNA (i.e., cases that went directly to surgery) and ROM after rFNA (Table 2). There were in total 8,083 cases in the Asian cohort and 3,914 cases in the non-Asian cohort.
In the direct to surgery cohorts, although the Asian group showed a trend towards a lower RR compared to the non-Asian cohort, this was not statistically significant [23.9% (95% CI, 18.0–29.8%) vs. 30.7% (95% CI, 21.1–40.2%), P=0.23]. In the Singapore series, the RR in directly resected cases was 40.5%.
The ROMs in both direct to surgery and post-rFNA cohorts paralleled each other, trending towards higher ROMs in the Asian groups, but the differences did not achieve statistical significance. The ROM in directly resected cases was 34.1% (95% CI, 18.2–49.9%) in the Asian cohort and 23.7% (95% CI, 16.1–31.4%) in the non-Asian cohort; P=0.25. The ROM of cases that were resected after rFNA was 40.2% (95% CI, 14.0–66.4%) in the Asian cohort and 28.1% (95% CI, 19.6–36.6%) in the non-Asian cohort; P=0.49.
In the Singapore series, the ROM in the direct to surgery cohort was 26.4%, and the ROM after rFNA was 33.3%.
AUS/FLUS with nuclear vs. architectural atypia: ROM and incidence
Eight Asian and 15 non-Asian series were included in the pooled analysis (Table 3); these included 1,112 cases in the Asian cohort and 1,563 cases in the non-Asian cohort. The ROM was higher in AUS-N than AUS-A; this association was apparent in both the Asian and the non-Asian series. In the Asian series, the ROMs of AUS-N and AUS-A were 49.6% (95% CI, 25.5–73.7%) and 17.0% (95% CI, 11.1–22.8%) respectively; P=0.049 (Figure 3A). This difference is also borne out in the non-Asian series, where the ROM of AUS-N vs. AUS-A was 45.8% (95% CI, 39.3–52.4%) vs. 24.0% (95% CI, 15.7–32.3%); P<0.001 (Figure 3B).
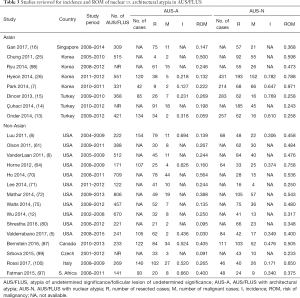
Full table

In Singapore, the ROM of AUS-N cases was significantly higher than that in AUS-A cases (36.8% vs. 14.7%, P=0.006).
Within the AUS-N cohort, there was no significant regional difference in ROM between Asian and non-Asian groups (P=0.73); and the same finding was obtained in the AUS-A cohort (P=0.43). Amongst the series that substratified AUS/FLUS by the presence of nuclear vs. architectural atypia, the actual incidences of AUS-N and AUS-A amongst all AUS/FLUS cases were documented in only four Asian (two from Korea and two from Turkey; n=1,566) and six non-Asian series (three from USA, one from Canada, one from Italy and one from South Africa; n=1,142). Nevertheless, there appeared to be a significant geographical difference in the incidence of AUS-A and AUS-N. The Asian cohort showed a higher incidence of AUS-N than the non-Asian cohort [70.3% (95% CI, 61.8–78.8%) vs. 33.5% (95% CI, 24.2–42.7%); P<0.001] (Figure 4A). This trend was reversed in AUS-A, where the Asian cohort demonstrated lower rates than the non-Asian cohort [22.3% (95% CI, 14.7–29.8%) vs. 57.6% (95% CI, 49.5–65.7%); P<0.001] (Figure 4B).

Incidence of papillary carcinoma and follicular neoplasms in surgically resected cases of AUS/FLUS
To further investigate for possible reasons for the regional differences in the incidences of AUS-A and AUS-N, we reviewed the prevalence of specific tumor subtypes in studies with at least 50 surgically resected cases with documented histologic diagnoses (Table 4). Nine Asian studies (one from Singapore, two from mainland China, one from Taiwan, three from Korea, and two from Turkey) and six non-Asian studies were reviewed (three from USA, one from Canada, one from Macedonia and one from Australia); which yielded a cumulative total of 2,318 cases and 1,076 cases in the Asian and non-Asian cohorts respectively. Amongst the Asian studies, the incidence of PTC (of any subtype) ranged from 16.1% to 65.7%, with the Singapore series exhibiting the lowest incidence. Excluding Singapore, the incidence ranged from 31.5% to 65.7%. Amongst the non-Asian studies, the incidence ranged from 9.3% to 45.5%. The pooled incidence of PTC trended higher in the Asian studies than the non-Asian studies [46.3% (95% CI, 34.8–57.9%) vs. 29.1% (95% CI, 17.3–40.9%), P=0.061] (Figure 5A). On exclusion of the results from the Singapore series, the PTC incidence in Asian vs. non-Asian studies was 50.2% vs. 29.1% respectively, and this difference reached statistical significance (P=0.009).
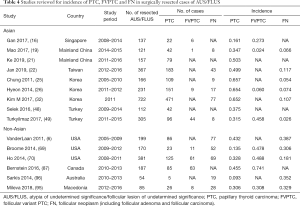
Full table
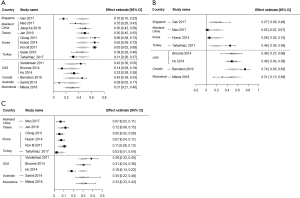
The subtypes of PTC were described in four Asian studies (one from Singapore, one from mainland China, one from Korea and one from Turkey) and four non-Asian studies (two from USA, one from Canada and one from Macedonia). The incidence of FVPTC as a proportion of all the PTCs ranged from 2.4% to 45.8% in Asian studies, whilst the range was 30.8% to 74.1% in non-Asian studies. The pooled incidence of FVPTC as a proportion of all the PTCs was lower in the Asian studies than the non-Asian studies [19.2% (95% CI, 3.8–34.7%) vs. 51.4% (95% CI, 32.9–69.8%), P=0.006] (Figure 5B).
For FNs (FA, FC), these were specifically mentioned in the histologic outcome in six Asian studies (one from mainland China, one from Taiwan, three from Korea, and one from Turkey); and five non-Asian studies (three from the USA, one from Macedonia and one from Australia) (6,19,22,25,26,32,49,69,70,95,96). Within Asian studies, the range of incidence was 2.6% to 11.7%, compared to 18.1% to 38.7% in the non-Asian studies (Figure 5C). The pooled incidence of FNs for Asian vs. non-Asian studies was 7.4% (95% CI, 4.0–10.7%) vs. 30.7% (95% CI, 21.2–40.1%); P<0.001.
Molecular studies in Asian vs. non-Asian centers
The type of molecular testing performed was noted when documented in the studies reviewed. It should be noted that the studies reviewed did not focus primarily on molecular risk stratification of indeterminate nodules, but, rather, on documenting the follow-up of indeterminate nodules.
Amongst the Asian centers, molecular testing was performed on indeterminate thyroid nodules in eight centers including those in mainland China (20) and in several university centers in Korea (7,24,26-30).
In both mainland China and Korea, the most frequent molecular test performed was mutational testing for BRAF (most frequently targeting the BRAF V600E mutation). Testing methods included Sanger sequencing (both mainland China and Korea), real-time PCR, dual-priming oligonucleotide based multiplex PCR (DPO-PCR), pyrosequencing and PCR-restriction fragment length polymorphism-based analysis (PCR-RFLP). With some of the more sensitive methods of testing, occasional false-positive cases were documented, with Park et al. reporting three false positive cases that, on histology, yielded a FA, Hurthle cell adenoma and a Hurthle cell carcinoma (7). Hence, the lowest positive predictive value for the BRAF V600E mutation was 90.6%, with the lowest reported specificity at 83.3% (7,20,24,26,28).
Gene array testing or multigene mutation testing was not reported in the Asian studies reviewed.
In the non-Asian papers, molecular tests were described in five studies, all from the USA (74,81-84). In contrast to Asian cohorts, all employed multigene or array-based analysis rather than focused BRAF mutation analysis. Tests included the Afirma gene expression classifier (GEC) (74,82,83), miRInform panel (81) and an oncogenic panel which included several RAS mutations, BRAF, PIK3CA and also RET/PTC and PAX8/PPARγ rearrangements performed by a centralized laboratory (84). None of the studies performed isolated BRAF mutation testing. The overall helpfulness of molecular testing in accurate risk stratification for surgery was modest. Seagrove-Guffey et al. found the oncogenic panel test unhelpful, with one NRAS-mutated case yielding a benign histologic outcome (adenomatoid nodule) whilst a negative case yielded PTC on histology (84). Valderrabano et al. tested mirINform on 19 AUS/FLUS cases, and found that none of the three excised cases yielded a positive result, whilst the negative predictive value (NPV) was 84% (81). They reported the results to be “worse than expected”, with a possible reason given that malignancies that were cytologically classified as AUS/FLUS tended to be FVPTC or other follicular-patterned cancers such as FC, which may have resulted in a higher proportion of negative tests. The differential prevalence of follicular-patterned neoplasms between Asian and non-Asian cohorts has been documented in the previous section.
Publication and small study bias
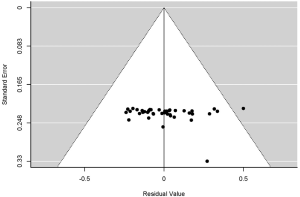
We noted funnel plot asymmetry in the studies included for the pooled analysis of incidence, overall RR and OROM of AUS/FLUS, as well as the incidence of AUS-A and FN (Figure S1). As most of the studies reviewed are observational and non-comparative, there were no “undesirable” results or characteristics like significance levels that may have caused publication bias. Hence, we surmise that the funnel plot asymmetry seen in these group of studies is possibly related to small study bias which could lead to exaggeration of effect. In the subset of studies that compared ROMs in cytomorphological subgroups (AUS-A vs. AUS-N), funnel plots showed no strong evidence of publication bias (Figure S2), with the rank correlation and regression tests confirming the absence of funnel plot asymmetry (P=0.056 and P=0.721).

Discussion
This review describes the geographic differences in the incidences, RRs, ROMs and diagnostic practices in the AUS/FLUS Bethesda category, focusing on Singapore, other Asian countries and non-Asian regions. Whilst meta-analytical methods for evaluating proportions were applied in this work, it should be noted that this is a broad approach that encompasses many aspects of practice. To capture a wider pool of studies, we have included reports that meet the adequacy criteria for the specific parameter under analysis only, recognizing and accepting the possibility of selective reporting.
Incidence of AUS/FLUS and RRs
AUS/FLUS is a challenging diagnostic category bearing a degree of inherent subjectivity. In our analysis, we found that the pooled incidence of AUS/FLUS was comparable between Asian and non-Asian series, at 8.8% and 9.1% respectively, which is close to the recommended prevalence by TBSRTC of less than 10%. In the reference study from Singapore which was conducted in our institution, the data showed an incidence of 6.4%, which is below the pooled Asian incidence. This may reflect the contribution of rapid on-site evaluation (ROSE) by the cytotechnologists as well as on-site provisional reporting in our pathologist-led FNA clinic—services which are widely utilized in our institution, but may not be uniformly practiced worldwide. ROSE serves to optimize specimen collection and processing, thereby reducing preparation artefacts and addressing adequacy issues upfront, both of which can contribute to the incidence of AUS/FLUS. The relatively low incidence may also reflect the reporting of thyroid FNAs by a smaller group of dedicated cytopathologists.
In Singapore, thyroid FNAs are performed mostly in the specialist setting, by clinicians (endocrinologists, surgeons, radiologists) and pathologists. In the reference study from our institution, the practice setting is that of a tertiary referral center with a multiracial patient population (chiefly comprising Chinese, Malays and Indians) with a minor proportion of overseas nationals hailing mostly from the Association of Southeast Asian Nations (ASEAN) countries. Thyroid FNAs are performed under direct palpation or ultrasound guidance. FNA diagnoses are made according to the framework of TBSRTC, with subsequent management guided by a combination of the Bethesda recommendations and clinical/imaging features.
With regard to the management of AUS/FLUS nodules, some regional differences surfaced in our pooled analysis. We found that the overall RR (with and without rFNA) was higher in the non-Asian cohort as compared to Asian series (43.4% vs. 33.2%, P=0.02), which is consistent with Vuong et al.’s recent meta-analysis (1). Although the overall RR was higher in the non-Asian cohort, we did not find any significant regional differences in the rate of rFNA and the RR of cases that went directly to surgery (i.e., did not undergo rFNA). Although the reasons underpinning the regional differences in RR is beyond the scope of this study, we surmise that the selection threshold for surgery is influenced by multiple considerations that may be region specific, such as cultural beliefs, health behaviors, healthcare costs and accessibility, all of which become particularly important in the management of indeterminate nodules. In addition, rates of surgery may also be influenced by regional variations in malpractice and litigation climates (101,102). The trend towards a more conservative approach in Asian countries may also in part reflect the move towards active surveillance of indeterminate nodules and low-risk carcinomas (103), for example, as documented in the guidelines from the Japanese Thyroid Association (104).
Interestingly, we found that in our institution in Singapore, the overall RR was 44.3%, which more closely approximated the non-Asian figure of 43.4%. A possible reason for this may be the fact that many of our clinicians undergo higher professional fellowship training in Western regions including the United Kingdom or the USA, and hence may be strongly influenced by Western practices and guidelines. The trend towards a lower threshold for surgery is also reflected in the incidence of the cases that underwent repeat FNA and those that underwent surgery directly. In the Singapore study, the rate of repeat FNA in AUS/FLUS nodules was 23.6%, lower than the pooled incidences of both the Asian (29.4%) and the non-Asian studies (28.0%). Conversely, the proportion of cases that underwent surgery without a repeat FNA was higher in Singapore (40.5%) than the pooled incidences of both the Asian (23.9%) and the non-Asian cohorts (30.7%). A possible reason for the tendency towards surgery could be the specialist setting and the relative accessibility to tertiary surgical services in our institution.
ROM
We found that the ROM was higher in the Asian cohort, at 43.2%, compared to 26.8% in the non-Asian cohort (P=0.005), whilst the OROMs were not statistically different. This geographical difference replicates Vuong et al.’s findings (1), and could be related to differences in true disease prevalence, divergent inclusion criteria for AUS/FLUS, discrepant thresholds for surgery or variable thresholds for histopathologic diagnosis of thyroid tumors. Given that we concurrently demonstrated a difference in the collective RR between Asian and non-Asian series, it is likely that the variation in the propensity for surgery accounts significantly for the regional difference in ROM.
With our finding that the RR of AUS nodules in Singapore more closely mirrors the non-Asian practice, it is unsurprising that the ROM of AUS nodules in Singapore is also closer to that of the non-Asian cohort (27% vs. 26.8%), and lower than the pooled Asian rate of 43.2%. This is also observed in the ROM in the direct to surgery cohort, which was 26.4%, and closer to the non-Asian rate of 23.7% than the Asian rate of 34.1%. This is likely to be related to the higher proportion of cases that underwent surgery directly without a repeat FNA.
Taking a broader view, in terms of the overall ROM among all Bethesda categories (I to VI), our single-year institutional audit revealed a ROM of 19.0% (unpublished data). This is relatively low and more comparable with non-Asian series which feature overall ROMs of 35.6% and 31.3% (1,105) as compared to 70.5% and 60.4% in Asian series (1,103). This again suggests that the practice in Singapore is more closely aligned to Western practice, with higher rates of surgery as compared to active surveillance.
AUS-N vs. AUS-A: ROM
When AUS/FLUS was substratified into AUS-N vs. AUS-A, our pooled analysis showed that in both Asian and non-Asian cohorts, the ROM was significantly higher in AUS-N compared to AUS-A cases [49.6% vs. 17.0% (Asian) and 45.8% vs. 24.0% (non-Asian)]. This trend has also been demonstrated in the Singapore cohort, and independently demonstrated in many studies (5-13,16). This common finding between the two regions is strong support for the rationale of subclassifying the AUS/FLUS Bethesda category based on the presence of nuclear atypia.
Additionally, in the Singapore cohort, we also found that there was a higher rate of benign diagnoses on rFNA of AUS-A nodules than rFNA of AUS-N (70.6% vs. 48.7%, P=0.05), which is consistent with the findings of others, for example, those documented by Rosario et al. in their Brazilian series (9,89).
Within our institution, the terminology “AUS” is used for nodules with focal atypical nuclear features (nuclear enlargement, grooves, abnormal chromatin pattern, nuclear crowding and poorly formed inclusions), whereas “FLUS” refers to cases with some degree of architectural atypia (microfollicles, trabeculae, or crowding) without significant nuclear atypia. Although this is not a practice that is recommended in TBSRTC, we have found that others have also documented a similar terminology, namely in Brazil and Turkey (9,14,15), while yet others have adopted other terminology to denote subcategories (5,7,8).
Incidence of AUS-N vs. AUS-A
Interestingly, in the ten studies with adequate data for this comparison, we found stark geographical differences in the incidences of the subgroups. The incidence of AUS-N appeared to be far higher amongst Asian series compared to non-Asian cohorts (70.3% vs. 33.5%, P<0.001). It is noted that some Asian countries such as Japan may have somewhat narrower inclusion criteria for the AUS category than TBSRTC, primarily selecting for cases with PTC-like nuclear atypia (AUS-N), whilst cases with architectural atypia are categorized into the suspicious for FN/FN (SFN/FN) category instead (104). Although specific data from Japan was not available for the current analysis, our pooled incidences of studies from other Asian countries (Korea and Turkey) did show a higher incidence of AUS-N as opposed to non-Asian countries. Indeed, corroborating this, we also found a significantly lower incidence of FNs in resected AUS/FLUS nodules in Asian countries (mainland China, Taiwan, Korea and Turkey) compared to non-Asian countries (7.4% vs. 30.7%). These findings together may reflect the trend amongst Asian countries to reserve the use of AUS/FLUS to cases with PTC-like nuclear atypia, and the tendency to categorize cases with indeterminate architectural atypia into the SFN/FN category instead.
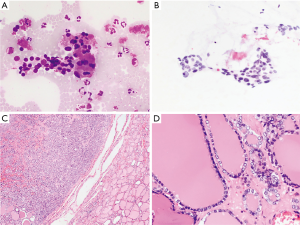
In view of the possible connection between cytomorphologic criteria of AUS-N and PTC, we examined the incidence of PTC in resected AUS/FLUS cases. Interestingly, we noticed that the Singapore cohort was an outlier with the lowest PTC incidence amongst the Asian studies (16.1%). After excluding the Singapore series, the PTC incidence in Asian vs. non-Asian studies was 50.2% vs. 29.1% respectively; P=0.009. Therefore, the relatively high incidence of PTC amongst resected cases may contribute to the higher prevalence of AUS-N cases in the Asian population. The low incidence of PTC in the Singapore AUS/FLUS cohort is intriguing, and raises the possibility of a difference in the true PTC disease prevalence of this population. Alternatively, this could potentially be accounted for by many PTC cases being cytologically classified into other Bethesda categories such as SM or Malignant. Another possible explanation is a high threshold for the histologic diagnosis of PTC, in particular, FVPTC, owing to the inherently subjective nature of interpretation of nuclear features. Figure 6 illustrates a case which was classified as “AUS” due to the presence of some nuclear atypia, and histologically diagnosed as FA, although some degree of nuclear pallor and enlargement was present.
Molecular practices
As we have shown in our results, there is a stark difference in the molecular approaches applied to AUS/FLUS nodules between Asian (chiefly mainland China and Korean) studies and those in the West (USA). Asian studies showed a much higher reliance on BRAF mutation testing (7,20,24,26-30), whilst Western studies favored broader multigene panel or array-based methods (74,81-84). None of the Asian or Western studies in the reviewed papers overlapped in their molecular interrogation methods.
We postulate that the preference for BRAF mutation testing in the Asian setting reflects the enrichment of PTCs in the AUS/FLUS category. This may be explained, at least in part, by the selection of cases with PTC-like nuclear atypia into this indeterminate category as discussed above. Furthermore, it appears that there is a higher rate of BRAF V600E mutation in Asian PTC cohorts than in Western cohorts, as illustrated in Korean centers where prevalence as high as 84% to 87% have been documented (106,107). This suggests that there is a greater proportion of classic PTC in Asian cohorts compared to FVPTC, given that the BRAF V600E mutation is more commonly detected in classical PTC. In support of this, the few studies that provided the breakdown of PTC variants showed that the percentage of FVPTCs amongst all PTCs was significantly lower in Asian series compared to non-Asian series (19.2% vs. 51.4%, P=0.006). This also bears out the point that BRAF mutations may be less helpful as a standalone test in non-Asian series, where not only is PTC less frequently encountered, but a greater proportion of the PTCs are FVPTCs, In Singapore, the BRAF mutation rate in PTC was 56% in a cohort from a single institution (108).
On the other side of the coin, RAS mutations feature more prominently in follicular-patterned neoplasms, which include FC, some FAs, NIFTPs and some FVPTCs. A loose comparison of the incidence of resected FNs within the AUS/FLUS category between Asian and non-Asian groups showed that these neoplasms featured more frequently in non-Asian than Asian cohorts (30.7% vs.7.4%, P<0.001). This ties in with our finding of higher AUS-A incidences in non-Asian cohorts compared to Asian series (57.6% vs. 22.3%, P<0.001). Altogether, this lends weight to the notion that Western practice as a whole have a greater tendency to classify cases with indeterminate architectural atypia as AUS/FLUS than the Asian practice. This may also partly explain why gene panel testing is the favored modality of molecular testing in the Western hemisphere.
Of course, cost and logistics also play a major role in the choice of molecular testing methods. Many of the commercially available gene panel tests and array-based tests were developed in the West, with limited accessibility in Asian countries.
In Singapore, molecular testing has not been validated in the local population and is not routinely performed in AUS/FLUS cases, however, individual tests may be performed on an ad-hoc basis. In such instances, the cost is considerable, as the aspirated material is usually transported to an accredited laboratory in the USA for testing, e.g., for the Thyroseq test. We have also found the results of repeat FNA to yield a more definitive cytologic diagnosis in 67.1% of cases, which is very helpful in the determination of the next management step.
Limitations
There are several limitations to this study. Firstly, it should be noted that the broad approach that we have adopted may have led to the inclusion of studies with selective reporting of some parameters. This may have introduced a further element of bias in addition to the inherent selection bias that accompanies retrospective studies, which makes up the majority of our data.
Secondly, there is a considerable amount of heterogeneity within each geographical cohort. Hence, the findings that we present here may be over generalized and not directly applicable to an individual institution’s practices. This emphasizes the need for follow-up studies in individual practices, as there may be considerable inter- and intra-regional variations in the application of TBSRTC, disease prevalence and management considerations.
Thirdly, we detected an element of possible small study bias in the pooled analysis of several parameters, namely incidence, RR and OROM of AUS/FLUS, as well as the incidence of AUS-A and FN.
One caveat to our review of Singapore’s practice is that it is drawn from a single tertiary institution’s experience, and hence may not be fully representative of the country’s practice as a whole. We also did not adjust for the impact of NIFTP on ROM as the number of cases in our series was too minimal for meaningful analysis (data not published). The impact of NIFTP has been comprehensively addressed by others and found to lower the ROM in indeterminate nodules to a lesser degree in Asian cohorts than in the West (2,3,109-113).
Conclusions
This review provides insights into regional differences in the diagnosis and management of AUS/FLUS nodules between Asian and non-Asian countries, with a focused comparison of the local experience in Singapore. We found that although the overall incidence of AUS/FLUS was comparable, there were significant differences in the RRs and ROMs between Asian and non-Asian cohorts, which may reflect different collective thresholds for surgery. There remains much heterogeneity within each of the regional cohorts, as exemplified by the Singapore experience, where the RR and ROM appear to parallel the non-Asian studies despite having a largely Asian population. This strongly supports the recommendation of TBSRTC to validate ROM estimates in individual practices, particularly in this AUS/FLUS category that is most susceptible to subjective interpretation.
Additionally, we found that the subgroup of AUS-N showed a significantly higher ROM than AUS-A in both Asian and non-Asian series. These corroborating findings in both regions provides convincing grounds for subclassifying the AUS/FLUS Bethesda category based on the presence of nuclear atypia.
Lastly, Asian series yielded a higher incidence of PTC on surgical follow-up, while FVPTC and FNs featured more prominently in non-Asian series. This finding parallels the higher AUS-N incidence in Asia and the higher AUS-A incidence in non-Asian countries, suggesting variations in the application of TBSRTC criteria within the AUS/FLUS category. These differential incidences may influence and, indeed, explain the molecular approaches adopted in the different regions, as they inform us on the cost-effectiveness and predictive value considerations. For example, performing a single gene (BRAF) mutation test may be highly cost-effective in the Asian setting due to the higher proportion of cases with PTC-like nuclear atypia amongst AUS/FLUS cases. Hence, an awareness of regional variations in the incidences of specific histologic subtypes and the nuances in the interpretation of TBSRTC will help streamline choices as we collectively move toward greater accessibility to molecular testing in the indeterminate Bethesda categories.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kennichi Kakudo) for the series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-555
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-555). The series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vuong HG, Ngo HTT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: A systematic review and meta‐analysis. Cancer Cytopathol 2020;128:238-49. [PubMed]
- Bychkov A, Keelawat S, Agarwal S, et al. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multi-institutional study in five Asian countries. Pathology 2018;50:411-7. [PubMed]
- Faquin WC, Wong LQ, Afrogheh AH, et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol 2016;124:181-7. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. J Am Soc Cytopathol 2017;6:217-22. [Crossref] [PubMed]
- Valderrabano P, Khazai L, Thompson ZJ, et al. Cancer Risk Stratification of Indeterminate Thyroid Nodules: A Cytological Approach. Thyroid 2017;27:1277-84. [Crossref] [PubMed]
- VanderLaan PA, Marqusee E, Krane JF. Usefulness of Diagnostic Qualifiers for Thyroid Fine-Needle Aspirations With Atypia of Undetermined Significance. Am J Clin Pathol 2011;136:572-7. [Crossref] [PubMed]
- Park HJ, Moon JH, Yom CK, et al. Thyroid ‘atypia of undetermined significance’ with nuclear atypia has high rates of malignancy and BRAF mutation. Cancer Cytopathol 2014;122:512-20. [Crossref] [PubMed]
- Luu MH, Fischer AH, Stockl TJ, et al. Atypical follicular cells with equivocal features of papillary thyroid carcinoma is not a low-risk cytologic diagnosis. Acta Cytol 2011;55:526-30. [Crossref] [PubMed]
- Rosario PW. Thyroid nodules with atypia or follicular lesions of undetermined significance (Bethesda Category III): importance of ultrasonography and cytological subcategory. Thyroid 2014;24:1115-20. [Crossref] [PubMed]
- Pagni F, Prada M, Goffredo P, et al. ‘Indeterminate for malignancy’ (Tir3/Thy3 in the Italian and British systems for classification) thyroid fine needle aspiration (FNA) cytology reporting: morphological criteria and clinical impact. Cytopathology 2014;25:170-6. [Crossref] [PubMed]
- Mosca L, Silva LFFD, Carneiro PC, et al. Malignancy rates for Bethesda III subcategories in thyroid fine needle aspiration biopsy (FNAB). Clinics (Sao Paulo) 2018;73:e370. [Crossref] [PubMed]
- Wu HH, Inman A, Cramer HM. Subclassification of “atypia of undetermined significance” in thyroid fine-needle aspirates: Atypia of Undetermined Significance. Diagn Cytopathol 2014;42:23-9. [Crossref] [PubMed]
- Onder S, Firat P, Ates D. The Bethesda system for reporting thyroid cytopathology: an institutional experience of the outcome of indeterminate categories. Cytopathology 2014;25:177-84. [Crossref] [PubMed]
- Çuhaci N, Arpaci D, Üçler R, et al. Malignancy rate of thyroid nodules defined as follicular lesion of undetermined significance and atypia of undetermined significance in thyroid cytopathology and its relation with ultrasonographic features. Endocr Pathol 2014;25:248-56. [Crossref] [PubMed]
- Dincer N, Balci S, Yazgan A, et al. Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology 2013;24:385-90. [PubMed]
- Gan TRX, Nga ME, Lum JHY, et al. Thyroid cytology-nuclear versus architectural atypia within the ‘Atypia of undetermined significance/follicular lesion of undetermined significance’ Bethesda category have significantly different rates of malignancy. Cancer Cytopathol 2017;125:245-56. [Crossref] [PubMed]
- Keelawat S, Rangdaeng S, Koonmee S, et al. Current Status of Thyroid Fine-Needle Aspiration Practice in Thailand. J Pathol Transl Med 2017;51:565-70. [Crossref] [PubMed]
- Thewjitcharoen Y, Butadej S, Nakasatien S, et al. Incidence and malignancy rates classified by The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) - An 8-year tertiary center experience in Thailand. J Clin Transl Endocrinol 2018;16:100175. [Crossref] [PubMed]
- Mao F, Xu HX, Zhao CK, et al. Thyroid imaging reporting and data system in assessment of cytological Bethesda Category III thyroid nodules. Clin Hemorheol Microcirc 2017;65:163-73. [Crossref] [PubMed]
- Zheng B, Zarka MA, Chen C, et al. The largest CAP-certified Chinese reference laboratory experience with the Bethesda system for reporting thyroid cytopathology: correlation with histologic and BRAF data. J Am Soc Cytopathol 2018;7:16-21. [Crossref] [PubMed]
- Ke J, Jianyong L, Ying L, et al. The use of The Bethesda System for Reporting Thyroid Cytopathology in a Chinese population: An analysis of 13 351 specimens. Diagn Cytopathol 2019;47:876-80. [Crossref] [PubMed]
- Jan IS, Lee YT, Wang CM, et al. The surgery and repeat aspiration outcomes of the atypia of undetermined significance/follicular lesion of undetermined significance category in The Bethesda System for Reporting Thyroid Cytopathology. Asian J Surg 2019;42:144-7. [Crossref] [PubMed]
- Satoh S, Yamashita H, Kakudo K. Thyroid Cytology: The Japanese System and Experience at Yamashita Thyroid Hospital. J Pathol Transl Med 2017;51:548-54. [Crossref] [PubMed]
- Kim SK, Hwang TS, Yoo YB, et al. Surgical Results of Thyroid Nodules according to a Management Guideline Based on the BRAF V600E Mutation Status. J Clin Endocrinol Metab 2011;96:658-64. [Crossref] [PubMed]
- Chung YS, Yoo C, Jung JH, et al. Review of atypical cytology of thyroid nodule according to the Bethesda system and its beneficial effect in the surgical treatment of papillary carcinoma. J Korean Surg Soc 2011;81:75-84. [Crossref] [PubMed]
- Hyeon J, Ahn S, Shin JH, et al. The prediction of malignant risk in the category ‘atypia of undetermined significance/follicular lesion of undetermined significance’ of the Bethesda System for Reporting Thyroid Cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol 2014;122:368-76. [Crossref] [PubMed]
- Yoo MR, Gweon HM, Park AY, et al. Repeat Diagnoses of Bethesda Category III Thyroid Nodules: What To Do Next? PloS One 2015;10:e0130138. [Crossref] [PubMed]
- Jung YY, Jung S, Lee HW, et al. Significance of Subcategory Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance Showing Both Cytologic and Architectural Atypia in Thyroid Aspiration Cytology. Acta Cytol 2015;59:370-6. [Crossref] [PubMed]
- Koh J, Moon HJ, Kim EK, et al. The 5-tiered categorization system for reporting cytology is sufficient for management of patients with thyroid nodules compared to the 6-tiered Bethesda system. Endocrine 2016;53:489-96. [Crossref] [PubMed]
- Kim SJ, Roh J, Baek JH, et al. Risk of malignancy according to sub-classification of the atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS) category in the Bethesda system for reporting thyroid cytopathology. Cytopathology 2017;28:65-73. [Crossref] [PubMed]
- Kim SD, Han SH, Jeong WJ, et al. Differences in Clinical Features Between Subcategories of ‘Atypia/Follicular Lesion of Undetermined Significance’. Endocr Pathol 2017;28:247-52. [Crossref] [PubMed]
- Kim M, Park HJ, Min HS, et al. The Use of the Bethesda System for Reporting Thyroid Cytopathology in Korea: A Nationwide Multicenter Survey by the Korean Society of Endocrine Pathologists. J Pathol Transl Med 2017;51:410-7. [Crossref] [PubMed]
- Hong SH, Lee H, Cho MS, et al. Malignancy Risk and Related Factors of Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance in Thyroid Fine Needle Aspiration. Int J Endocrinol 2018;2018:4521984. [Crossref] [PubMed]
- Mondal SK, Sinha S, Basak B, et al. The Bethesda system for reporting thyroid fine needle aspirates: A cytologic study with histologic follow-up. J Cytol 2013;30:94-9. [Crossref] [PubMed]
- Mehra P, Verma AK. Thyroid cytopathology reporting by the bethesda system: a two-year prospective study in an academic institution. Patholog Res Int 2015;2015:240505. [Crossref] [PubMed]
- Garg S, Desai NJ, Mehta D, et al. To Establish Bethesda System for Diagnosis of Thyroid Nodules on the Basis of Fnac with Histopathological Correlation. J Clin Diagn Res 2015;9:EC17-21. [PubMed]
- Arul P, Akshatha C, Masilamani S. A study of malignancy rates in different diagnostic categories of the Bethesda system for reporting thyroid cytopathology: An institutional experience. Biomed J 2015;38:517-22. [Crossref] [PubMed]
- Mahajan S, Srinivasan R, Rajwanshi A, et al. Risk of Malignancy and Risk of Neoplasia in the Bethesda Indeterminate Categories: Study on 4,532 Thyroid Fine-Needle Aspirations from a Single Institution in India. Acta Cytol 2017;61:103-10. [Crossref] [PubMed]
- Kumari KA, Jadhav PD, Prasad C, et al. Diagnostic Efficacy of Ultrasound-Guided Fine Needle Aspiration Combined with the Bethesda System of Reporting. J Cytol 2019;36:101-5. [Crossref] [PubMed]
- Naz S, Hashmi AA, Khurshid A, et al. Diagnostic accuracy of Bethesda system for reporting thyroid cytopathology: an institutional perspective. Int Arch Med 2014;7:46. [Crossref] [PubMed]
- Ozluk Y, Pehlivan E, Gulluoglu MG, et al. The use of the Bethesda terminology in thyroid fine-needle aspiration results in a lower rate of surgery for nonmalignant nodules: a report from a reference center in Turkey. Int J Surg Pathol 2011;19:761-71. [Crossref] [PubMed]
- Ustün H, Astarcı HM, Altunkaya C, et al. Fine-needle aspiration of follicular patterned lesions of the thyroid: diagnosis, management, and follow-up according to thyroid Bethesda system. Acta Cytol 2012;56:361-9. [Crossref] [PubMed]
- Firat P, Cochand-Priollet B. The Bethesda system for reporting thyroid fine needle aspiration cytology: a study comparing the results of two centers from two different countries. Ann Pathol 2012;32:e29-34, 415-20.
- Tepeoğlu M, Bilezikçi B, Bayraktar SG. A histological assessment of the Bethesda system for reporting thyroid cytopathology (2010) abnormal categories: a series of 219 consecutive cases. Cytopathology 2014;25:39-44. [Crossref] [PubMed]
- Muratli A, Erdogan N, Sevim S, et al. Diagnostic efficacy and importance of fine-needle aspiration cytology of thyroid nodules. J Cytol 2014;31:73-8. [Crossref] [PubMed]
- Gocun PU, Karakus E, Bulutay P, et al. What is the malignancy risk for atypia of undetermined significance? Three years’ experience at a university hospital in Turkey. Cancer Cytopathol 2014;122:604-10. [Crossref] [PubMed]
- Kuru B, Atmaca A, Kefeli M. Malignancy rate associated with Bethesda category III (AUS/FLUS) with and without repeat fine needle aspiration biopsy. Diagn Cytopathol 2016;44:394-8. [Crossref] [PubMed]
- Selek A, Cetinarslan B, Kıvrakoğlu E, et al. Histologic outcome of thyroid nodules with repeated diagnosis of atypia in thyroid fine-needle aspiration biopsy. Future Oncol 2016;12:801-5. [Crossref] [PubMed]
- Turkyilmaz S, Ulusahin M, Celebi B, et al. Thyroid nodules classified as atypia or follicular lesions of undetermined significance deserve further research: Analysis of 305 surgically confirmed nodules. Cytopathology 2017;28:391-9. [Crossref] [PubMed]
- Öcal B, Korkmaz MH, Yılmazer D, et al. The Malignancy Risk Assessment of Cytologically Indeterminate Thyroid Nodules Improves Markedly by Using a Predictive Model. Eur Thyroid J 2019;8:83-9. [Crossref] [PubMed]
- Mufti ST, Molah R. The bethesda system for reporting thyroid cytopathology: a five-year retrospective review of one center experience. Int J Health Sci (Qassim) 2012;6:159-73. [Crossref] [PubMed]
- Al-Abbadi MA, Shareef SQ, Ali JA, et al. Application of the Bethesda System for Reporting Thyroid Cytopathology in the Eastern Province of Saudi Arabia: phase I pilot retrospective analysis. Acta Cytol 2013;57:481-8. [Crossref] [PubMed]
- Alabdulqader NA, Shareef SQ, Ali JA, et al. Application of the Bethesda System for Reporting Thyroid Cytopathology in the Eastern Province of Saudi Arabia: A Follow-Up Study. Acta Cytol 2015;59:233-8. [Crossref] [PubMed]
- Al Dawish MA, Robert AA, Muna A, et al. Bethesda System for Reporting Thyroid Cytopathology: A three-year study at a tertiary care referral center in Saudi Arabia. World J Clin Oncol 2017;8:151-7. [Crossref] [PubMed]
- Hirsch D, Robenshtok E, Bachar G, et al. The Implementation of the Bethesda System for Reporting Thyroid Cytopathology Improves Malignancy Detection Despite Lower Rate of Thyroidectomy in Indeterminate Nodules. World J Surg 2015;39:1959-65. [Crossref] [PubMed]
- Ronen O, Cohen H, Abu M. Review of a single institution’s fine needle aspiration results for thyroid nodules: Initial observations and lessons for the future. Cytopathology 2019;30:468-74. [Crossref] [PubMed]
- Sinna EA, Ezzat N. Diagnostic accuracy of fine needle aspiration cytology in thyroid lesions. J Egypt Natl Canc Inst 2012;24:63-70. [Crossref] [PubMed]
- Al-Abbadi MA, Shareef SQ, Yousef MM, et al. A follow-up study on thyroid aspirates reported as atypia of undetermined significance/follicular lesion of undetermined significance and follicular neoplasm/suspicious for follicular neoplasm: A multicenter study from the Arabian Gulf region. Diagn Cytopathol 2017;45:983-8. [Crossref] [PubMed]
- Jo VY, Stelow EB, Dustin SM, et al. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol 2010;134:450-6. [Crossref] [PubMed]
- Rabaglia JL, Kabbani W, Wallace L, et al. Effect of the Bethesda system for reporting thyroid cytopathology on thyroidectomy rates and malignancy risk in cytologically indeterminate lesions. Surgery 2010;148:1267-72; discussion 1272-3. [Crossref] [PubMed]
- Olson MT, Clark DP, Erozan YS, et al. Spectrum of risk of malignancy in subcategories of ‘atypia of undetermined significance’. Acta Cytol 2011;55:518-25. [Crossref] [PubMed]
- Bongiovanni M, Crippa S, Baloch Z, et al. Comparison of 5-tiered and 6-tiered diagnostic systems for the reporting of thyroid cytopathology. Cancer Cytopathol 2012;120:117-25. [Crossref] [PubMed]
- Chen JC, Pace SC, Chen BA, et al. Yield of repeat fine-needle aspiration biopsy and rate of malignancy in patients with atypia or follicular lesion of undetermined significance: the impact of the Bethesda System for Reporting Thyroid Cytopathology. Surgery 2012;152:1037-44. [Crossref] [PubMed]
- Horne MJ, Chhieng DC, Theoharis C, et al. Thyroid follicular lesion of undetermined significance: Evaluation of the risk of malignancy using the two-tier sub-classification. Diagn Cytopathol 2012;40:410-5. [Crossref] [PubMed]
- Harvey AM, Mody DR, Amrikachi M. Thyroid fine-needle aspiration reporting rates and outcomes before and after Bethesda implementation within a combined academic and community hospital system. Arch Pathol Lab Med 2013;137:1664-8. [Crossref] [PubMed]
- Nagarkatti SS, Faquin WC, Lubitz CC, et al. Management of thyroid nodules with atypical cytology on fine-needle aspiration biopsy. Ann Surg Oncol 2013;20:60-5. [Crossref] [PubMed]
- Olson MT, Boonyaarunnate T, Aragon Han P, et al. A tertiary center’s experience with second review of 3885 thyroid cytopathology specimens. J Clin Endocrinol Metab 2013;98:1450-7. [Crossref] [PubMed]
- Theoharis C, Adeniran AJ, Roman S, et al. The impact of implementing The Bethesda System for reporting of thyroid FNA at an academic center. Diagn Cytopathol 2013;41:858-63. [Crossref] [PubMed]
- Broome JT, Cate F, Solorzano CC. Utilization and impact of repeat biopsy for follicular lesion/atypia of undetermined significance. World J Surg 2014;38:628-33. [Crossref] [PubMed]
- Ho AS, Sarti EE, Jain KS, et al. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS). Thyroid 2014;24:832-9. [Crossref] [PubMed]
- Lee B, Smola B, Roh MH, et al. The impact of using the Bethesda System for reporting thyroid cytology diagnostic criteria on the follicular lesion of undetermined significance category. J Am Soc Cytopathol 2014;3:131-6. [Crossref] [PubMed]
- Mathur A, Najafian A, Schneider EB, et al. Malignancy risk and reproducibility associated with atypia of undetermined significance on thyroid cytology. Surgery 2014;156:1471-6. [Crossref] [PubMed]
- McElroy MK, Mahooti S, Hasteh F. A single institution experience with the new bethesda system for reporting thyroid cytopathology: correlation with existing cytologic, clinical, and histological data. Diagn Cytopathol 2014;42:564-9. [Crossref] [PubMed]
- Sullivan PS, Hirschowitz SL, Fung PC, et al. The impact of atypia/follicular lesion of undetermined significance and repeat fine-needle aspiration: 5 years before and after implementation of the Bethesda System. Cancer Cytopathol 2014;122:866-72. [Crossref] [PubMed]
- Walts AE, Mirocha J, Bose S. Follicular lesion of undetermined significance in thyroid FNA revisited. Diagn Cytopathol 2014;42:18-22. [Crossref] [PubMed]
- Deniwar A, Hambleton C, Thethi T, et al. Examining the Bethesda criteria risk stratification of thyroid nodules. Pathol Res Pract 2015;211:345-8. [Crossref] [PubMed]
- Kantola S, Virani N, Haus C, et al. Prospective evaluation of impact of using the Bethesda System for Reporting Thyroid Cytopathology: an institutional experience. J Am Soc Cytopathol 2015;4:25-9. [Crossref] [PubMed]
- Brandler TC, Aziz MS, Coutsouvelis C, et al. Young investigator challenge: Atypia of undetermined significance in thyroid FNA: Standardized terminology without standardized management--a closer look at repeat FNA and quality measures. Cancer Cytopathol 2016;124:37-43. [Crossref] [PubMed]
- Krauss EA, Mahon M, Fede JM, et al. Application of the Bethesda Classification for Thyroid Fine-Needle Aspiration: Institutional Experience and Meta-analysis. Arch Pathol Lab Med 2016;140:1121-31. [Crossref] [PubMed]
- Shrestha RT, Hennessey JV. Cytologic subclassification of atypia of undetermined significance may predict thyroid nodules more likely to be malignant at surgery. Diagn Cytopathol 2016;44:492-8. [Crossref] [PubMed]
- Valderrabano P, Leon ME, Centeno BA, et al. Institutional prevalence of malignancy of indeterminate thyroid cytology is necessary but insufficient to accurately interpret molecular marker tests. Eur J Endocrinol 2016;174:621-9. [Crossref] [PubMed]
- Guo A, Kaminoh Y, Forward T, et al. Fine Needle Aspiration of Thyroid Nodules Using the Bethesda System for Reporting Thyroid Cytopathology: An Institutional Experience in a Rural Setting. Int J Endocrinol 2017;2017:9601735. [Crossref] [PubMed]
- Deaver KE, Haugen BR, Pozdeyev N, et al. Outcomes of Bethesda categories III and IV thyroid nodules over 5 years and performance of the Afirma gene expression classifier: A single-institution study. Clin Endocrinol (Oxf) 2018;89:226-32. [Crossref] [PubMed]
- Seagrove-Guffey MA, Hatic H, Peng H, et al. Malignancy rate of atypia of undetermined significance/follicular lesion of undetermined significance in thyroid nodules undergoing FNA in a suburban endocrinology practice: A retrospective cohort analysis. Cancer Cytopathol 2018;126:881-8. [Crossref] [PubMed]
- Bresler A, Mehta V, Schiff BA, et al. Comparison of Bethesda cytopathology classification to surgical pathology across racial-ethnic groups. Head Neck 2019;41:2340-5. [Crossref] [PubMed]
- Williams BA, Bullock MJ, Trites JR, et al. Rates of thyroid malignancy by FNA diagnostic category. J Otolaryngol Head Neck Surg 2013;42:61. [Crossref] [PubMed]
- Bernstein JM, Shah M, MacMillan C, et al. Institution-specific risk of papillary thyroid carcinoma in atypia/follicular lesion of undetermined significance: Risk of PTC in Atypia/Follicular Lesion of Undetermined Significance. Head Neck 2016;38:E1210-5. [Crossref] [PubMed]
- Erivwo P, Ghosh C. Atypia of Undetermined Significance in Thyroid Fine-Needle Aspirations: Follow-Up and Outcome Experience in Newfoundland, Canada. Acta Cytol 2018;62:85-92. [Crossref] [PubMed]
- Rosario PW, Calsolari MR. Importance of cytological subclassification of thyroid nodules with Bethesda category III cytology (AUS/FLUS) into architectural atypia only and nuclear atypia: A prospective study. Diagn Cytopathol 2017;45:604-7. [Crossref] [PubMed]
- Reuters KB, Mamone MCOC, Ikejiri ES, et al. Bethesda Classification and Cytohistological Correlation of Thyroid Nodules in a Brazilian Thyroid Disease Center. Eur Thyroid J 2018;7:133-8. [Crossref] [PubMed]
- Ratour J, Polivka M, Dahan H, et al. Diagnosis of follicular lesions of undetermined significance in fine-needle aspirations of thyroid nodules. J Thyroid Res 2013;2013:250347. [Crossref] [PubMed]
- Stanek-Widera A, Biskup-Frużyńska M, Zembala-Nożyńska E, et al. Clinical importance of follicular lesion of undetermined significance (diagnostic category III according to Bethesda System) diagnosed from Fine-Needle Aspiration Biopsy. Endokrynol Pol 2016;67:12-6. [Crossref] [PubMed]
- Estrada Muñoz L, Díaz Del Arco C, Ortega Medina L, et al. Thyroid Atypia/Follicular Lesion of Undetermined Significance: Attitudes towards the Diagnosis of Bethesda System III Nodules. Acta Cytol 2017;61:21-6. [Crossref] [PubMed]
- Paajanen I, Metso S, Jaatinen P, et al. Thyroid FNA diagnostics in a real-life setting: Experiences of the implementation of the Bethesda system in Finland. Cytopathology 2018;29:189-95. [Crossref] [PubMed]
- Mileva M, Stoilovska B, Jovanovska A, et al. Thyroid cancer detection rate and associated risk factors in patients with thyroid nodules classified as Bethesda category III. Radiol Oncol 2018;52:370-6. [Crossref] [PubMed]
- Sarkis LM, Norlen O, Aniss A, et al. The Australian experience with the Bethesda classification system for thyroid fine needle aspiration biopsies. Pathology 2014;46:592-5. [Crossref] [PubMed]
- Fatman L, Michelow P. Thyroid cytopathology with an emphasis on the ‘atypical cells of uncertain significance’ category: a 3-year audit with cytohistologic correlation. Acta Cytol 2015;59:17-25. [Crossref] [PubMed]
- Ryu YJ, Jung YS, Yoon HC, et al. Atypia of undetermined significance on thyroid fine needle aspiration: surgical outcome and risk factors for malignancy. Ann Surg Treat Res 2014;86:109-14. [Crossref] [PubMed]
- Srbova L, Gabalec F, Ryska A, et al. Results of retrospective classification of thyroid FNAs according to the Bethesda system: would this have improved accuracy? Cytopathology 2015;26:231-7. [Crossref] [PubMed]
- Rossi M, Lupo S, Rossi R, et al. Proposal for a novel management of indeterminate thyroid nodules on the basis of cytopathological subclasses. Endocrine 2017;57:98-107. [Crossref] [PubMed]
- Labarge B, Walter V, Lengerich EJ, et al. Evidence of a positive association between malpractice climate and thyroid cancer incidence in the United States. PLoS One 2018;13:e0199862. [Crossref] [PubMed]
- Kakudo K, Bychkov A, Abelardo A, et al. Malpractice Climate Is a Key Difference in Thyroid Pathology Practice Between North America and the Rest of the World. Arch Pathol Lab Med 2019;143:1171. [Crossref] [PubMed]
- Kakudo K, Higuchi M, Hirokawa M, et al. Thyroid FNA cytology in Asian practice-Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017;28:455-66. [Crossref] [PubMed]
- Kakudo K, Kameyama K, Miyauchi A, et al. Introducing the reporting system for thyroid fine-needle aspiration cytology according to the new guidelines of the Japan Thyroid Association. Endocr J 2014;61:539-52. [Crossref] [PubMed]
- Bongiovanni M, Spitale A, Faquin WC, et al. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol 2012;56:333-9. [Crossref] [PubMed]
- Kim SK, Kim DL, Han HS, et al. Pyrosequencing analysis for detection of a BRAFV600E mutation in an FNAB specimen of thyroid nodules. Diagn Mol Pathol 2008;17:118-25. [Crossref] [PubMed]
- Kim SK, Song KH, Lim SD, et al. Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid 2009;19:137-41. [Crossref] [PubMed]
- Goh X, Lum J, Yang SP, et al. BRAF mutation in papillary thyroid cancer-Prevalence and clinical correlation in a South-East Asian cohort. Clin Otolaryngol 2019;44:114-23. [Crossref] [PubMed]
- Bongiovanni M, Faquin W, Giovanella L, et al. Impact of non-invasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP) on risk of malignancy in patients undergoing lobectomy/thyroidectomy for suspicious for malignancy or malignant fine-needle aspiration cytology findings: a systematic review and meta-analysis. Eur J Endocrinol 2019;181:389-96. [Crossref] [PubMed]
- Zhou H, Baloch ZW, Nayar R, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): Implications for the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). Cancer Cytopathol 2018;126:20-6. [Crossref] [PubMed]
- Ruanpeng D, Cheungpasitporn W, Thongprayoon C, et al. Systematic review and meta-analysis of the impact of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features (NIFTP) on cytological diagnosis and thyroid cancer prevalence. Endocr Pathol 2019;30:189-200. [Crossref] [PubMed]
- Higuchi M, Hirokawa M, Kanematsu R, et al. Impact of the modification of the diagnostic criteria in the 2017 Bethesda System for Reporting Thyroid Cytopathology: a report of a single institution in Japan. Endocr J 2018;65:1193-8. [Crossref] [PubMed]
- Vuong HG, Tran TTK, Bychkov A, et al. Clinical impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the risk of malignancy in the bethesda system for reporting thyroid cytopathology: a meta-analysis of 14,153 resected thyroid nodules. Endocr Pract 2019;25:491-502. [Crossref] [PubMed]


