
An affordable immunohistochemical approach to estimate the prevalence of BRAFV600E in large cohort studies—establishing the baseline rate of BRAF mutation in an institutional series of papillary thyroid carcinoma from Thailand
Introduction
Thyroid cancer is the most common endocrine malignancy whose incidence has been increasing dramatically over the past three decades due to increased recognition of thyroid nodules harboring carcinoma (1,2). It is known to be the fastest-growing cancer in women worldwide, particularly in economically developed countries where high resolution imaging is widely available (1). The rate of thyroid cancer in Thailand is similar to average Asian and worldwide rates—according to the recent estimates, thyroid carcinoma is one of the leading malignancies in Thai women, occupying the fourth rank by prevalence (3).
Papillary thyroid carcinoma (PTC) is the major histological subtype, accounting for approximately 85% of all thyroid cancers (4), which also holds true for Thailand (5). Compared to other human malignancies, PTC carries an excellent overall prognosis with a 10-year survival rate approaching 95% (4,6). There are several risk factors associated with the development of PTC, of which exposure to ionizing radiation has been the well-documented environmental cause of PTC (2). Other factors include genetic predisposition, hormonal influence, dietary components, such as iodine, nitrates, and alcohol, and more modifiers (7-9).
BRAFV600E mutation is the most common somatic driver event in PTC (4,10). BRAFV600E is detected in almost half of PTC cases in Western cohorts (45–50%) (11). In contrast, Asian series of PTC have much higher variation of BRAF incidence, which was reported ranging 31–87% (12). The presence of BRAFV600E is known to be clinically relevant in terms of diagnosis, adverse prognosis, and treatment strategy (10,11,13), therefore establishing a rate of BRAF on the national and even institutional level is of practical significance. For example, preoperative BRAF testing in cytologic specimens is much effective in areas with high prevalence of BRAF mutation (14,15). To our knowledge, there are no well-established datasets or publications in international peer-reviewed journals available on the prevalence of BRAF mutation in Thai PTC till date.
DNA-based molecular testing methods, especially Sanger sequencing has been widely acknowledged as the gold standard for the detection of point mutations in solid tumors. However, molecular techniques are relatively expensive, time-consuming, and have certain limitations regarding specimen quality, sampling adequacy, tumor heterogeneity, and more (16,17). A novel approach to detect BRAFV600E by means of mutation-specific monoclonal antibody VE1 has been recently established (18). Immunohistochemistry (IHC) is a rapid, simple and cost-effective method that doesn't require the establishment of the molecular laboratory. Numerous studies performed on PTC, colorectal carcinoma, melanoma, and other BRAF-mutant tumors reported excellent concordance between VE1 IHC and molecular genotyping and suggested that VE1 IHC is a reliable method that can be used as an alternative to BRAF sequencing (18-24).
Considering a need to establish a baseline rate of BRAF mutation in PTC from Thailand, we designed an affordable approach combining advantages of VE1 IHC and tissue microarray (TMA). The latter technology allows multiple tissue samples to be arrayed into a single paraffin block, thus significantly reducing the costs of reagents (25). In addition to the main purpose above, we also aimed to validate the performance of VE1 immunostaining against molecular genotyping and to evaluate clinical relevance of BRAF mutation in the Thai series of PTC. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-388).
Methods
Case selection
King Chulalongkorn Memorial Hospital (KCMH), Bangkok, one of the largest tertiary referral centers for thyroid cancer in Thailand, served as a reference institution. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). We conducted retrospective cross-sectional study with the approval from the Human Research Ethics Committee of KCMH and Chulalongkorn University Institutional Review Board (IRB No. 050/61).
Based on electronic database search, there were 1,038 patients who underwent thyroidectomy at KCMH from January 2007 to December 2017. Among them, 733 cases were diagnosed by surgical pathologists as PTC. All specimen types (total thyroidectomy, hemithyroidectomy, subtotal or near total thyroidectomy, lobectomy, and tumor excision) of primary PTC were included. From 733 PTC cases, formalin-fixed paraffin-embedded (FFPE) blocks of 113 cases and clinical information of 21 cases were missing from our archive and database, respectively. In addition, 70 cases had tumor size insufficient for TMA preparation (<4 mm), and 53 cases had repeated hospital number, i.e., re-operated for the same tumor. Based on our inclusion and exclusion criteria, the total number of PTC cases employed for this study was 467. The case selection process is summarized in Figure 1.
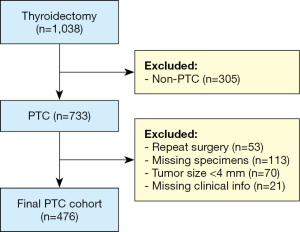
Tumor characteristics and clinical information of each patient were retrieved from the hospital pathology database, including patient’s gender and age, histological variants, tumor size, laterality, multifocality, extrathyroidal extension, margins status, lymphovascular invasion, presence of Hashimoto’s thyroiditis, lymph node metastasis, distant metastasis, and pathological staging. FFPE tissue samples were retrieved from archives of the Department of Pathology, KCMH. All pathology slides were reviewed by two pathologists (S.C. and A.B.) and classified as per the terminology and diagnostic criteria of the WHO classification of Tumors of Endocrine Organs (2). Cancer staging was done as per the American Joint Committee on Cancer (AJCC) staging system, 8th edition (26).
Tissue microarray construction
TMA MASTER (3D HISTECH Ltd.; Budapest, Hungary) tissue microarrayer was used for TMA construction. All hematoxylin and eosin (H&E) stained slides of PTC were reviewed and the slide with a representative tumor was selected from each case. A single representative area of tumor was encircled on the conventional H&E slide and the area corresponding to the selected area on the FFPE block was marked with the felt marker. One core from each case was then cored out with a 2-mm diameter needle and transferred to a recipient paraffin block for tissue microarray construction. A distance of 1-mm was kept between each core.
VE1 immunohistochemistry
We performed immunohistochemistry for VE1 on 4 µm-thick TMA sections using an automated Ventana BenchMark Ultra autostainer (Ventana Medical Systems, Tucson, AZ). Tissues sections were incubated with the anti-BRAFV600E (VE1) mouse monoclonal antibody (Ventana Medical Systems, catalog number 790-4855) for 32 min at 37 °C. Immunoreactivity of VE1 was visualized using an OptiView DAB IHC detection kit (Ventana Medical Systems) and then counterstained with Hematoxylin II and Bluing Reagent for 8 min. and 4 min., respectively. We used human tonsil tissue as a negative control tissue for each staining run. The VE1 immunoreactivity was assessed by two pathologists (S.C. and S.K.) using the H-scoring system and discrepancies were discussed till the consensus was reached. H-score is a semi-quantitative system which includes both the proportion (0–100%) and intensity of positive cells (0, absent; 1+, weak; 2+, moderate; 3+, strong staining). The range of H-score was obtained by combining intensity and proportion scores and hence, final scores obtained were ranging from 0 to 300, as described previously (16,27). Based on our previous study using the similar method (16), where H-score of >10 was considered as positive for mutation, we found out that there was no significant association between cutoff point of H-score against the BRAF mutation. Therefore, in the current study, positive H-score (any cytoplasmic positivity) was considered as indicative of BRAF on immunostaining.
Sanger sequencing of BRAF exon 15
Bidirectional Sanger sequencing of BRAF mutations was done for all cases from the pilot cohort at the outside facility (Department of Hospital Pathology, College of Medicine, The Catholic University, Seoul, Korea). Total DNA was extracted from 10 µm thick paraffin-embedded whole tissue sections using RecoverAll™ Total Nucleic Acid Isolation Kit (Life Technologies, Carlsbad, CA, USA) as per the manufacturer’s instruction. PCR reaction was performed using a primer pair (forward, 5'-TCATAATGCTTGCTCTGATAGGA-3' and reverse, 5'-GGCCAAAAATTTAATCAGTGGA-3'). Sanger sequencing was performed using the same primers and BigDye Terminator sequencing kit (Applied Biosystems, Carlsbad, CA, USA) on a 3730xl DNA analyzer (Applied Biosystems) as previously described (16).
Statistical analysis
All the statistical analyses were performed by using SPSS 22.0 software (IBM, Armonk, NY, USA). By considering the direct sequencing as the gold standard for the detection of BRAFV600E mutation, we assessed the sensitivity, specificity, likelihood ratios, negative predictive value and positive predictive value of VE1 IHC in detecting BRAFV600E mutation in PTC. The agreement between IHC and direct sequencing results were evaluated using the Kappa coefficient (κ). Pearson’s chi-square-test and Fisher exact test were used to assess the differences between categorical variables as appropriate. Student t-test was used to compare the means of continuous variables. Data are reported as mean ± SD or number (%). The P value of less than 0.05 was considered statistically significant.
Results
Analytical performance of VE1 in the pilot cohort
Based on sample size calculation, the minimum required sample size of our pilot cohort was 63 cases, which was further expanded to 100 PTCs (unselected continuous cohort 2009–2012) with an intention to increase the reliability of the pilot study. All PTC cases were initially assessed on H&E staining to assure the presence of tumor. VE1 immunostaining of different intensities, including weak (1+), moderate (2+) and strong (3+) intensities were observed in the cytoplasm of the tumor cells (Figure 2). Majority of the cases showed homogenous cytoplasmic staining. However, few cases showed heterogeneous distribution of stain with variable intensities and proportions. Hence, application of the H-scoring system was considered the optimal choice for evaluating staining in the pilot cohort.
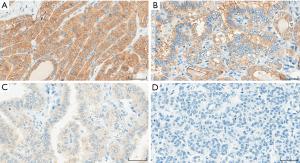
Of 100 PTC cases employed for pilot study, 69/100 (69%) were positive for VE1 expression. Direct sequencing of BRAF exon 15 detected BRAFV600E mutation in 68/100 (68%) cases and 32/100 (32%) cases were of wild type. There were 5 discordant cases. 2 cases were positive for mutation by direct sequencing but negative by VE1 (false negative), and 3 cases were negative for mutation by direct sequencing but VE1 positive on immunostaining (false positive). All of the discordant cases were of classic variant.
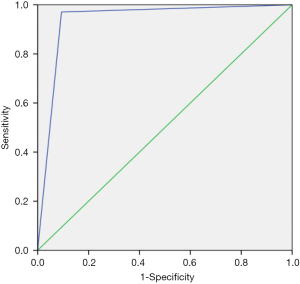
By considering the direct sequencing as the gold standard method, VE1 IHC showed sensitivity and specificity of 97.1% and 90.1% respectively. The receiver operating characteristic (ROC) curve demonstrated high validity of VE1 IHC in detecting BRAFV600E mutation in PTC specimens and found to be a comparable method with direct sequencing. This was corroborated with 93.1% area under the curve (Figure 3). The positive (9.80) and negative (0.03) likelihood ratios corresponded to the interpretation of VE1 IHC as “often useful” and “very useful”, respectively, test for detecting the mutation. The positive and negative predictive values were 95.7% and 93.5% respectively. The VE1 IHC and direct sequencing results for detecting BRAFV600E mutation in PTC tissue showed almost perfect agreement (κ=0.884) with an overall percentage agreement of 95.0%.
Prevalence of BRAFV600E mutation in Thai PTC
We performed VE1 IHC to all of the subsequent PTC cases involved in our study as an alternative to direct sequencing. Since VE1 IHC showed excellent analytical performance in detecting BRAFV600E mutation, any cytoplasmic immunoexpression (positive H-score) was further considered as equivalent to BRAFV600E mutation. BRAFV600E mutation was detected in 286/476 (60.9%), by VE1 IHC.
Clinical and pathological characteristics
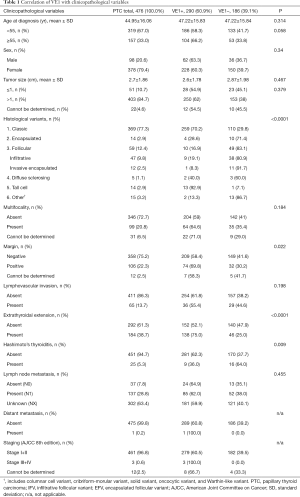
Of the 476 included cases, 378/476 (79.4%) cases were females and only 98/476 (20.6%) cases were males. The age ranged from 8 to 87 years with a mean age of 44.9 years. Surgical interventions included lobectomy, total thyroidectomy, completion thyroidectomy, subtotal thyroidectomy, and excision, which were performed in 132/476 (27.7%), 230/476 (48.3%), 7/476 (1.5%), 87/476 (18.3%) and 5/476 (1.1%) cases respectively. Right lobe of the thyroid gland was the most common location of the tumor comprising 184/476 (38.7%) of the total cases, followed by left lobe comprising 134/476 (28.2%) of total cases. The tumor size ranged from 0.4 cm to 11 cm in the greatest dimension, with a mean size of 2.7 cm. Of the total cases involved, 25/476 (5.3%) had Hashimoto’s thyroiditis as the background of thyroid cancer. Most of PTCs were of classic variant 369/476 (77.5%), followed by follicular variant 59/476 (12.4%), and tall cell variant 14 /476 (2.9%). Among the follicular variant PTCs, 47 (9.8%) and 12 (2.5%) cases were infiltrative follicular and invasive encapsulated follicular subtypes, respectively. The vast majority of cases (82.8%) belonged to the clinical stage I (AJCC 8th edition). Interestingly, this was largely contributed not by the tumor size (10.7% of microcarcinomas in the whole cohort) but rather by the age < 55 years (67% patients).
Correlation of BRAF mutation with clinicopathological variables
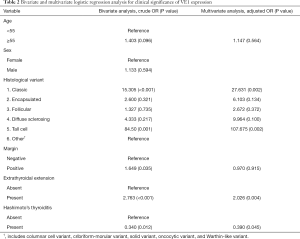
The correlation between clinicopathological variables and BRAFV600E mutation in PTC are presented in Table 1. BRAFV600E mutation was mostly seen in older patients, tumors of larger size, and multifocality. However, these variables showed no significant association with BRAF mutation. The study showed no significant difference between the BRAFV600E rates in male and female patients. Microcarcinomas showed the rate of BRAFV600E (57%) comparable to that in the whole PTC cohort. Although the majority of the histological types involved in this study was of conventional variant, BRAFV600E was frequently seen in tall cell variant (13/14, 92.9%), followed by classic variant (259/368, 70.2%). The influence of histological variants is well demonstrated by the higher rate of BRAFV600E in the pilot series compared to the whole cohort (69% vs. 60.9%), which was influenced by the higher prevalence of classic PTC in the former set. Eight of 9 cases with perineural invasion were positive for BRAFV600E mutation (88.9%). Only one case had distant metastasis (bone metastasis) at the time of diagnosis, which turned out to be positive for BRAFV600E mutation in the primary tumor. On univariate analysis, BRAFV600E was significantly associated with margin positivity (P=0.022), extrathyroidal extension (P<0.0001), classic variant (P<0.001), and absence of Hashimoto’s thyroiditis (P=0.009).
Full table
Variables showing a tendency of association with VE1 (P<0.25) in the univariate analysis were included in the multivariate logistic regression model. Bivariate analysis showed association of BRAFV600E mutation with older age of patients, absence of Hashimoto’s thyroiditis, perineural invasion, extrathyroidal extension, margin positivity, classic variant and tall cell variants of PTC. Multivariate analysis showed association of BRAFV600E mutation with classic and tall cell variants of PTC, extrathyroidal extension, and absence of Hashimoto’s thyroiditis (Table 2). The ROC curve analysis further demonstrated that the combined pathological variables listed above have a fair chance of predicting the presence of BRAFV600E mutation in our series, which was corroborated by 75.03% area under the curve.
Full table
Discussion
The BRAFV600E mutation has been reported to be prevalent in PTC and is associated with adverse prognostic factors in this tumor (4,6,10). It can also act as a diagnostic marker for PTC among various types of thyroid cancer (14). In this series, we report our institutional experience on the detection of BRAFV600E mutation on TMA of PTC specimens by using VE1 IHC and also the association of BRAFV600E mutation with clinicopathological variables.
To validate the performance of VE1 IHC before using it for detection of BRAFV600E mutation in this study, we initially selected 100 PTC cases to compare IHC results with the direct sequencing study. As compared to direct sequencing, VE1 IHC showed a sensitivity of 97.1% and the specificity of 90.1%, which is concordant with previous studies (18-24). There were 5 discordant cases in our pilot cohort, which included 2 cases negative for BRAFV600E by VE1 IHC but positive by direct sequencing (false negative result) and 3 cases positive for BRAFV600E by VE1 IHC but was negative by direct sequencing (false positive result). We repeated IHC on the whole tissue section of all these discordant cases but the results were persistently the same as initially.
Although direct sequencing has been widely regarded as the gold standard method for detection of point mutation (16,28), it has been reported to have relatively lower sensitivity, requiring higher percentage of tumor cells within the samples (28) and produced more false negative results when it is used as a solo validation method (16). In our case, the tumor size of one of the initial false positive cases was 0.4 cm and moreover, we couldn't adopt additional molecular workup for our discordant cases since resolving discordant cases was not our objective. However, we believe that employing cases with adequate tumor size and using a combination of molecular methods for validation might produce lower false negative rates. The possible explanation for VE1 IHC false negative in our study could be due to the loss of mutation antigen. It has been reported that the long-term storage of tissue sections suffers from loss of antigenicity (29,30). Another cause of false negative is heterogeneity of tumor lesion (31,32). This may explain why VE1 immunoreactivity is not detected in some TMA tissue samples (31,33). Nevertheless, VE1 IHC in our study produced excellent analytical performance with overall percentage agreement of 95.0% in detecting BRAFV600E mutation in PTC as compared to direct sequencing.
We performed clinicopathological correlations with BRAFV600E mutation (VE1+) and found that BRAFV600E was associated with classic and tall cell variants of PTC, margin positivity (on univariate and bivariate analysis), presence of extrathyroidal extension, and absence of Hashimoto’s thyroiditis. Several studies have shown the role of BRAFV600E in tumor aggressiveness and inferior clinical outcome in PTC patients (10,28). Our findings were in accordance with previous publications, which defined extrathyroidal extension, tall cell morphology, and positive surgical margin as predictors of aggressive behavior of PTC, while the association of Hashimoto’s thyroiditis with outcome of differentiated thyroid cancer in currently debated (34). BRAF mutation has been recently introduced to the risk stratification chart of patients with differentiated thyroid cancer as a factor conveying a higher chance of tumor recurrence (34,35). Since our access to clinical data was limited only to the records provided in the laboratory information system, we could not evaluate further correlation with such important endpoints of PTC outcome as recurrence and mortality in this series. Additional studies are warranted to prove association of BRAFV600E mutation with outcome of thyroid cancer in Thai patients.
This is the first large-scale study on BRAF rate in PTC from Thailand, and we believe that our series collected at the major tertiary referral cancer center could be representative of the whole country. A similar approach has been successfully employed recently to establish a baseline rate of human papillomavirus in head-neck cancer (36). Our study reports the rate of BRAFV600E mutation in Thai PTC as 60.9%. This is relatively lower than BRAFV600E prevalence in PTC reported by the close neighbors like Vietnam (83%) and the Philippines (70.6%); however, number of the cases enrolled in these studies was as low as 53 and 17, respectively—this is definitely not sufficient to draw meaningful conclusions about the nationwide rate (37,38). Reports from other Asian countries, such as Japan, and South Korea are much more abundant and consistently showed the high prevalence of BRAFV600E mutation (> 70%) in PTC (12,39).
It is not surprising to see such a scarce amount of data from the Southeast Asian countries, because most of them cope with limited resources. One of the limitations of our study was we could not adopt additional molecular study for our discordant cases in pilot cohort and also we could not perform molecular study to all of the cases involved due to limited budget and time period but the major purposes of our project was to develop a low-cost testing alternative to estimate the prevalence of BRAFV600E in large cohort studies. IHC to substitute genotyping was one way to reduce costs. Importantly, this step required initial validation with a reference molecular test in a well-powered pilot series. Another approach to significantly minimize expenses was a using of small-sized specimens instead of whole-tissue sections (25,40). Finally, by combining VE1 IHC and TMA, we could afford performing a large cohort study in limited resource settings. We believe that our approach can serve as a model for other institutions.
Conclusions
BRAFV600E mutation was detected in 60.9% of Thai PTC and it was associated with several aggressive clinicopathological variables of thyroid cancer. VE1 IHC is a reliable method and may serve as alternative to direct sequencing for the detection of mutation within resource-limited and healthcare-cost-containment environments. A combination of mutation-specific IHC and TMA allows conducting large cohort studies more labor-saving and cost-efficiently.
Acknowledgments
We express our gratitude to Mrs. Kanista Keetacheeva and Ms. Jutamas Wongphoom for their excellent technical assistance. The authors thank to Ms. Atthanee Chiyaphan for the statistical advice.
Funding: This study was supported by Ratchadapisek Sompoch Endowment Fund, Faculty of Medicine, Chulalongkorn University; (RA 62/076), Bangkok, Thailand.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kennichi Kakudo) for the series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-388
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-388
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-388
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-388). The series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” was commissioned by the editorial office without any funding or sponsorship. CKJ serves as an unpaid editorial board member of Gland Surgery from July 2019 to June 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Human Research Ethics Committee of King Chulalongkorn Memorial Hospital, Bangkok and Chulalongkorn University Institutional Review Board (IRB No. 050/61).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wiltshire JJ, Drake TM, Uttley L, et al. Systematic Review of Trends in the Incidence Rates of Thyroid Cancer. Thyroid 2016;26:1541-52. [Crossref] [PubMed]
- Lloyd RV, Osamura RY, Kloppel G, et al. WHO Classification of Tumors of Endocrine Organs 4th ed. International Agency for Research on Cancer (IARC): Lyon, France; 2017.
- Tangjaturonrasme N, Vatanasapt P, Bychkov A. Epidemiology of head and neck cancer in Thailand. Asia Pac J Clin Oncol 2018;14:16-22. [Crossref] [PubMed]
- Fagin JA, Wells SA Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med 2016;375:1054-67. [Crossref] [PubMed]
- Bychkov A. A pathologist's perspective on thyroid cancer trends in Thailand. Cancer Epidemiol 2017;47:133-4. [Crossref] [PubMed]
- Giordano TJ. Genomic Hallmarks of Thyroid Neoplasia. Annu Rev Pathol 2018;13:141-62. [Crossref] [PubMed]
- Matsuse M, Takahashi M, Mitsutake N, et al. The FOXE1 and NKX2-1 loci are associated with susceptibility to papillary thyroid carcinoma in the Japanese population. J Med Genet 2011;48:645-8. [Crossref] [PubMed]
- Orim F, Bychkov A, Shimamura M, et al. Thyrotropin signaling confers more aggressive features with higher genomic instability on BRAF(V600E)-induced thyroid tumors in a mouse model. Thyroid 2014;24:502-10. [Crossref] [PubMed]
- Rogounovitch TI, Bychkov A, Takahashi M, et al. The common genetic variant rs944289 on chromosome 14q13.3 associates with risk of both malignant and benign thyroid tumors in the Japanese population. Thyroid 2015;25:333-40. [Crossref] [PubMed]
- Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007;28:742-62. [Crossref] [PubMed]
- Xing M, Alzahrani AS, Carson KA, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. Jama 2013;309:1493-501. [Crossref] [PubMed]
- Bychkov A. Prevalence of BRAF(V600E) mutation in Asian patients with thyroid cancer. Malays J Pathol 2017;39:95-6. [PubMed]
- Li C, Lee KC, Schneider EB, et al. BRAFV600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab 2012;97:4559-70. [Crossref] [PubMed]
- Kim SW, Lee JI, Kim JW, et al. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab 2010;95:3693-700. [Crossref] [PubMed]
- Kleiman DA, Sporn MJ, Beninato T, et al. Preoperative BRAF(V600E) mutation screening is unlikely to alter initial surgical treatment of patients with indeterminate thyroid nodules: a prospective case series of 960 patients. Cancer 2013;119:1495-502. [Crossref] [PubMed]
- Choden S, Keelawat S, Jung CK, et al. VE1 Immunohistochemistry Improves the Limit of Genotyping for Detecting BRAFV600E Mutation in Papillary Thyroid Cancer. Cancers 2020;12:596. [Crossref] [PubMed]
- Giannini R, Ugolini C, Lupi C, et al. The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumor foci in multifocal papillary thyroid carcinoma. J Clin Endocrinol Metab 2007;92:3511-6. [Crossref] [PubMed]
- Capper D, Preusser M, Habel A, et al. Assessment of BRAFV600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 2011;122:11-9. [Crossref] [PubMed]
- Bullock M, O'Neill C, Chou A, et al. Utilization of a MAB for BRAF(V600E) detection in papillary thyroid carcinoma. Endocr Relat Cancer 2012;19:779-84. [Crossref] [PubMed]
- Ilie MI, Lassalle S, Long-Mira E, et al. Diagnostic value of immunohistochemistry for the detection of the BRAF(V600E) mutation in papillary thyroid carcinoma: comparative analysis with three DNA-based assays. Thyroid 2014;24:858-66. [Crossref] [PubMed]
- Koperek O, Kornauth C, Capper D, et al. Immunohistochemical detection of the BRAFV600E-mutated protein in papillary thyroid carcinoma. Am J Surg Pathol 2012;36:844-50. [Crossref] [PubMed]
- Routhier CA, Mochel MC, Lynch K, et al. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAFV600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum Pathol 2013;44:2563-70. [Crossref] [PubMed]
- Na JI, Kim JH, Kim HJ, et al. VE1 immunohistochemical detection of the BRAFV600E mutation in thyroid carcinoma: a review of its usefulness and limitations. Virchows Arch 2015;467:155-68. [Crossref] [PubMed]
- Pyo JS, Sohn JH, Kang G. BRAF Immunohistochemistry Using Clone VE1 is Strongly Concordant with BRAF(V600E) Mutation Test in Papillary Thyroid Carcinoma. Endocr Pathol 2015;26:211-7. [Crossref] [PubMed]
- Hutchins G, Grabsch HI. How to make tissue microarrays. Diagnostic Histopathology 2018;24:127-35. [Crossref]
- Amin MB, Edge S, Greene F, et al. editors. AJCC Cancer Staging Manual. Eight edition ed. Springer International Publishing, 2017.
- Bychkov A, Sampatanukul P, Shuangshoti S, et al. TROP-2 immunohistochemistry: a highly accurate method in the differential diagnosis of papillary thyroid carcinoma. Pathology 2016;48:425-33. [Crossref] [PubMed]
- Kim JK, Seong CY, Bae IE, et al. Comparison of Immunohistochemistry and Direct Sequencing Methods for Identification of the BRAF(V600E) Mutation in Papillary Thyroid Carcinoma. Ann Surg Oncol 2018;25:1775-81. [Crossref] [PubMed]
- DiVito KA, Charette LA, Rimm DL, et al. Long-term preservation of antigenicity on tissue microarrays. Lab Invest 2004;84:1071-8. [Crossref] [PubMed]
- Karlsson C, Karlsson MG. Effects of long-term storage on the detection of proteins, DNA, and mRNA in tissue microarray slides. J Histochem Cytochem 2011;59:1113-21. [Crossref] [PubMed]
- de Biase D, Cesari V, Visani M, et al. High-sensitivity BRAF mutation analysis: BRAFV600E is acquired early during tumor development but is heterogeneously distributed in a subset of papillary thyroid carcinomas. J Clin Endocrinol Metab 2014;99:E1530-8. [Crossref] [PubMed]
- Fugazzola L, Muzza M, Pogliaghi G, et al. Intratumoral Genetic Heterogeneity in Papillary Thyroid Cancer: Occurrence and Clinical Significance. Cancers (Basel) 2020;12:382. [Crossref] [PubMed]
- Ghossein RA, Katabi N, Fagin JA. Immunohistochemical detection of mutated BRAFV600E supports the clonal origin of BRAF-induced thyroid cancers along the spectrum of disease progression. J Clin Endocrinol Metab 2013;98:E1414-21. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Kakudo K, Bychkov A, Bai Y, et al. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol Int 2018;68:641-64.
- Nopmaneepaisarn T, Tangjaturonrasme N, Rawangban W, et al. Low prevalence of p16-positive HPV-related head-neck cancers in Thailand: tertiary referral center experience. BMC Cancer 2019;19:1050. [Crossref] [PubMed]
- Vuong HG, Kondo T, Oishi N, et al. Genetic alterations of differentiated thyroid carcinoma in iodine-rich and iodine-deficient countries. Cancer Med 2016;5:1883-9. [Crossref] [PubMed]
- Espiritu GAM, Malana JT, Dumasis A, et al. High Preponderance of BRAFV600E Mutation in Papillary Thyroid Carcinoma Among Filipinos: A Clinicopathologic Study. J Glob Oncol 2019;5:1-6. [Crossref] [PubMed]
- Song YS, Lim JA, Park YJ. Mutation Profile of Well-Differentiated Thyroid Cancer in Asians. Endocrinol Metab (Seoul) 2015;30:252-62. [Crossref] [PubMed]
- Bychkov A, Jain D. Multiple sections per slide for immunohistochemistry: A cost-effective alternative for research in resource-limited settings. Anal Quant Cytopathol Histpathol 2018;40:211-2.


