
Prediction of level V metastases in papillary thyroid microcarcinoma: a single center analysis
Introduction
The incidence of thyroid cancer (TC) has increased dramatically around the world in recent decades. Papillary thyroid carcinoma (PTC) is the most common type of TC, accounting for nearly 85% (1-3). With the development of diagnostic technology, an increasingly number of papillary thyroid microcarcinoma (PTMC) was detected, which defined as tumor with a maximum diameter ≤10 mm (4,5). The majority of PTMCs have an indolent disease course and excellent prognosis. However, some PTMCs with lateral lymph node metastasis (LNM) or distant metastasis show an aggressive course, which can cause poor disease-free survival (6-8).
To obtain local control of lateral LNM, the American Thyroid Association (ATA) (9) recommended therapeutic lateral neck dissection for PTC patients with clinically suspected LNM (N1b). In general, the extent of therapeutic lateral neck dissection encompasses levels II–V. However, it is controversial whether routine level V dissection is necessary in N1b PTC patients. Some scholars (10-12) disagree that level V dissection should be routinely conducted in treatment of N1b PTC patients, because of the rate of level V metastasis is significantly low compared to levels II-IV and routine level V dissection may increase associated postoperative morbidities (shoulder dysfunction, sensory changes), the hospital time and cost. Conversely, conservative lateral neck dissection may increase the risk of disease-specific mortality and overall locoregional recurrence because some occult LNM cannot be found by preoperative imaging examination. There has no consensus whether routine level V dissection for N1b PTC patients and this topic remains widely debated (13-15). Therefore, an effective and accuracy treatment way is critical.
Compare with PTC, PTMC patients tend to skip metastasis and exhibit more frequent multifocality (16,17). To our knowledge, there have few studies to investigate the predictive factors for level V metastasis. Moreover, the benefits and clinical risks of routine level V dissection are uncertainty. Therefore, this study aimed to explore the incidence, pattern and clinical predictive factors for level V metastasis in N1b PTMC patients and attempt to provide a rational management of lateral neck dissection in treatment for N1b PTMC patients. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-232).
Methods
Patient selection
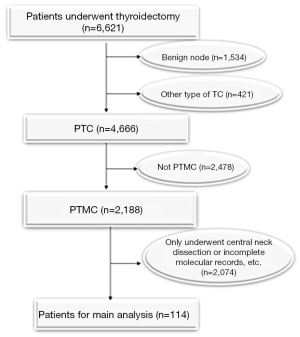
This study retrospectively reviewed the clinical records of 6,621 patients who underwent thyroidectomy from September 2016 to July 2019 at our center. After strict inclusion and exclusion criteria, a total of 114 patients met the requirements (Figure 1). Inclusion criteria as follows: (I) N1b PTMC patients with complete medical records; (II) underwent total thyroidectomy with bilateral central neck dissection and modified radical neck dissection (MRND), and postoperative histopathology confirmed PTMC with lateral LNM. The exclusion criteria for this study were as follows: (I) history of thyroidectomy; (II) benign node and other types of TC, such as follicular cancer, medullary cancer and anaplastic cancer etc.; (III) distant metastasis; (IV) selective lateral neck dissection or level I dissection. No robotic or endoscopic thyroidectomy was conducted in the enrolled patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xiangya Hospital Central South University (No.: 201901006) and informed consent was taken from all the patients.
Treatment strategy
Physical examination, ultrasound (US), enhanced computed tomography (CT) and fine needle aspiration biopsy (FNAB) were routinely performed in this study to assess the cervical lymph nodes and thyroid nodules before surgery. MRND was performed after total thyroidectomy and central neck dissection, the extent of MRND encompasses levels II–V, with sparing the sternocleidomastoid muscle, internal jugular vein, spinal accessory nerve and other important structures (external jugular vein, Musculus omohyoideus and cervical plexus) (18). The lateral compartment delimited inferiorly to the subclavian vein, superiorly to the sublingual nerve, and lateral to the anterior edge of the trapezius muscle. All dissected lymph node specimens were recorded according to the cervical regions, and sent to the Department of Pathology at Xiangya Hospital, the histopathological evaluation of these specimens was conducted by two or more pathologists with at least 8 years of experience and diagnosed more than 400 PTC cases. Thyroid stimulating hormone (TSH) suppression therapy with or without radioactive iodine 131 was conducted after the initial surgery. In this study, all patients received regular follow-up within 1 month and then every 3 months for the first year. Physical examination, US, thyroglobulin (TG), TGAb, TSH, free triiodothyronine, free thyroxine were measured at every outpatient visit.
Clinicopathological variables analyzed
The clinicopathological variables included sex, age, solitary/multifocal lesions, primary tumor size, bilaterality, capsular invasion, Hashimoto’s thyroiditis (HT), extrathyroidal extension (ETE), lymph nodes were harvested from the neck dissection specimens by different cervical regions. To distinguish multifocality from bilaterality, bilaterality is defined as the presence of carcinoma in both thyroid lobes, multifocality means two or more tumors lesion in the thyroid lobes. Gross ETE was regarded as the tumor penetrates through the capsule and invades the subcutaneous soft tissues, esophagus, trachea or recurrent laryngeal nerves (19). According to the 8th edition of TNM staging of TC issued by the AJCC in January 2018, we use 55 years old as the age cut-off value.
Statistical analyses
Statistical analysis was performed using SPSS (22.0 version). Continuous variables were expressed as the mean ± standard deviation, categorical variables were expressed as percentage (%) and frequency, the fisher exact test and chi-square test or were used for categorical variables. Multivariate logistic regression analysis was carried out on the significant clinical indicators. The sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV) and likelihood ratio (LR) were estimated to assess the predictive value. The ROC analysis was used to determine the predictive value of significant variable for level V metastasis. P<0.05 was considered to indicate statistical significance.
Results
Patient demographics
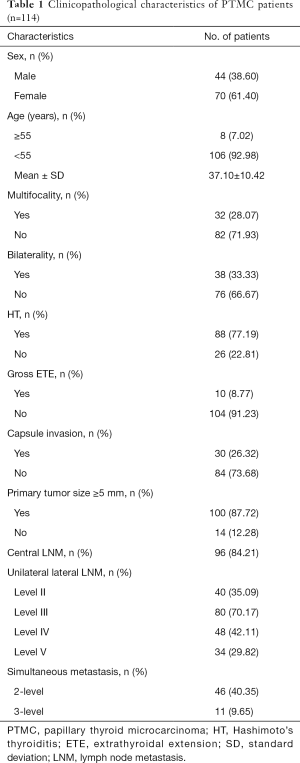
There were 114 patients involved in this study, including 44 males and 70 females. The average age was 37.10±10.42 years (range, 21–66 years), and 8 (7.02%) were older than 55 years old. The mean size of the primary tumor was 7.2 mm (range, 1–10 mm), 100 (87.72%) of patients with primary tumor size greater than 5 mm. Among all patients, 32 cases were multifocality, 10 (8.77%) patients exhibited gross ETE, and 30 (26.32%) patients presented capsule invasion. HT was detected in 88 (77.19%) patients and bilaterality was detected in 38 (33.33%) patients. All patients underwent central neck dissection and MRND, central LNM was found in 96 (84.21%) cases. metastatic lymph nodes were detected in 40 (35.09%), 80 (70.17%) and 48 (42.11%) of the level II, III and IV dissections, respectively. Simultaneous 2-level involvement of lymphatic metastases was observed in 46 (40.35%) cases and simultaneous 3-level metastasis was seemed in 11 (9.65%) cases (Table 1).
Full table
Predictors of level V LNM
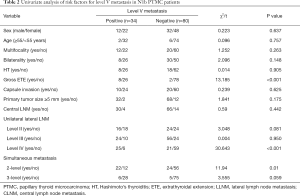
The overall and occult rate of level V metastasis were 29.82% (34/114) and 7.02% (8/114), respectively. Tables 2 and 3 show the relationships between several clinicopathologic factors and level V metastasis in the 114 N1b PTMC patients who underwent MRND. Univariate analysis showed that level V metastasis was significantly associated with gross ETE (P<0.001), level IV metastasis (P<0.001) and 2-level simultaneous metastasis (P=0.01). However, sex (P=0.637), age (P=0.757), multifocality (P=0.263), bilaterality (P=0.148), HT (P=0.905), capsule invasion (P=0.625), primary tumor size ≥5 mm (P=0.175), central LNM (P=0.442), level II metastasis (P=0.081) level III metastasis (P=0.950), 3-level simultaneous metastasis (P=0.059) were not significantly related with level V metastasis. In multivariate analysis, only gross ETE (OR =11.916; 95% CI, 1.404–102.19; P=0.023) and level IV metastasis (OR =8.497; 95% CI, 2.119–34.065; P=0.03) served as independent predictors of level V metastasis in N1b PTMC patients.
Full table

Full table
Predictive value for level V metastasis
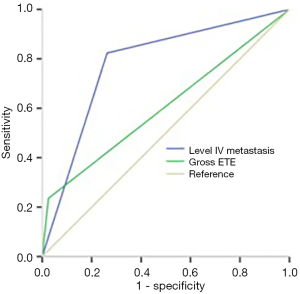
N1b PTMC patients with gross ETE to predict level V metastasis showed a sensitivity of 25.3%, a specificity of 97.5%, an accuracy of 82.69%, a PPV of 80%, an NPV of 75%, a +LR of 9.41and a −LR of 0.78. Compared with the predictive value of gross ETE, the sensitivity, specificity, accuracy, PPV, NPV, +LR and −LR for level IV metastasis to predict level V metastasis were 82.40%, 73.80%, 76.32%, 57.04%, 90.77%, 3.14 and 0.24, respectively (Table 4). Meantime, the AUC of gross ETE was lower than level IV metastasis (0.605 vs. 0.781, P=0.041) (Figure 2).

Full table
Discussion
With the development of high-resolution ultrasonography and improved health awareness, the detection incidence and rate of PTMC have greatly increased in the latest decade (8). The majority of PTMCs have an indolent disease course and excellent prognosis, active surveillance of low-risk PTMC patients is suggested as an alternative to surgical treatment to reduce associated postoperative morbidities. Nevertheless, immediate dissection of thyroid and cervical lymph nodes is required for N1b PTMC patients (6). Multiple guidelines recommend therapeutic lateral neck dissection if lateral LNM are confirmed by radiological evaluation or clinical examination (9,20). However, the optimal lateral neck dissection range still have no definitive guidelines, at present, MRND (level II to level V) has been generally accepted. The complications associated with MRND and clinical outcomes have been reported in many studies, but the effects are still debated (21,22). This is partly because of the desire to decrease the associated postoperative morbidities, especially injury for cervical plexus and spinal accessory nerve, and partly because of the lack of enough evidence that lateral LNM impact overall survival. Whether level V dissection should be included in N1b PTMC patients is a challenge for surgeon. Therefore, we designed this study to explore the clinical predictive factors for level V metastasis and attempt to provide a rational management of lateral neck dissection in treatment for N1b PMTC patients.
In our study, the overall and occult rate of level V metastasis were 29.82% (34/114) and 7.02% (8/114), respectively. Which is similar with the findings of previous study (10-12,14,21,23,24), and the overall incidence of lateral LNM in levels II, III and IV were 35.09%, 70.17% and 42.11%, respectively. Level III was the most common regions of lateral LNM, followed by levels IV and II. Therefore, selective neck dissection encompasses levels III–IV was necessary in N1b PTMC patients. Farrag and colleagues (25) retrospective reviewed 53 N1b PTC patients who underwent therapeutic lateral neck dissection and found lateral LNM were most common involved with levels III, II-A, and IV, the extent of lateral neck dissection at least including levels III, II-A, and IV. Another study (21) concluded that routine MRND is not required for all N1b patients as LNM to levels V was uncommon and recommended levels III–IV lymphadenectomy only. Further levels were only to be dissected under the evidence of clinical or radiographical examination. From the above mentioned information, it is no undoubted that lateral neck dissection can be accuracy planned in N1b PTMC patients to address the levels involved.
Whether level V should be encompassed in the lateral neck dissection is still widely debated. Kim et al. (11) opposed to routine level V dissection among the treatment of N1b PTC patients due to the relatively low rates of level V metastasis and recurrence. Meantime, level V dissection increased the risk of morbidities like “shoulder syndrome” and had a negative impact on their quality of life. Xue et al. (12) also agreed that it is unnecessary to routine level V dissection in N1b PTMC patients. Besides, routine level V dissection can lead to the higher cost and the longer hospital time. But Kupferman et al. (26) reported that the rate of level V metastasis was 53% and routine level V dissection should be performed in well-differentiated thyroid carcinoma patients with lateral neck metastasis. Javid et al. (22) believed that omitting levels V among lateral neck dissection can increase the risk for recurrent and persistent disease, formal MRND is necessary to avoid the morbidity of reoperation. Battoo et al. (13) thought there need a homogeneous study to clarify the necessity of level V dissection among N1b PTMC patients. In our study, the incidence of level V metastasis was low and it is unnecessary to undergo routine level V dissection without clinical level V metastasis. At present, the evidence is equivocal whether to routinely dissect level V or not. Moreover, preoperative US and CT have limited ability to identify the presence of level V metastasis. Therefore, we further explored the predictive factors of level V metastasis to help surgeons make a right decision in level V dissection.
In our study, we demonstrated gross ETE (OR =11.916; 95% CI, 1.404–102.19; P=0.023) and level IV metastasis (OR =8.497; 95% CI, 2.119–34.065; P =0.03) were the independent predictors of level V metastasis in N1b PTMC patients, which firstly reported in PTMC patients. Therefore, we suggest level V dissection is necessary in N1b PTMC patients with level IV metastasis or gross ETE, which was compatible with previous studies (27). Several studies (12,23,28) have indicated that multiple-level simultaneous metastasis was an independent predictor for level V metastasis. Our study also demonstrated that level V metastasis was related with 2-level simultaneous metastasis (P=0.01). However, multiple-level simultaneous metastasis was not an independent predictors of level V metastasis, this difference may be associated with population enrollment and primary tumor size. Besides, we explored the predictive value of gross ETE and level IV metastasis. The sensitivity, specificity, accuracy of gross ETE and level IV metastasis in predicting the level V metastasis were 25.3% vs. 82.4%, 97.5% vs. 73.8%, 82.69% vs. 76.32%, respectively. The AUC of gross ETE was lower than level IV metastasis (0.605 vs. 0.781, P=0.041). Therefore, compared with gross ETE, level IV metastasis is superior in predicting level V metastasis.
Our study has some potential limitations. First, this is a single center study. A study based on prospective and multicenter is more powerful and reproducible. Second, the number of patients was not large enough, only 114 patients being ultimately enrolled. A larger population study is needed to further confirm our results. Third, level V nodes were not routinely divided into sublevels Va and Vb. Therefore, we could not analysis the rate of metastasis levels Va and Vb separately. Fourth, our center was not routinely recorded some information in the pathological report, such as lymph nodal size, extra nodal extension, histological subtype and distant metastasis. Fifth, although all patients received regular follow-up, we failed to record all patients’ clinical follow-up data, so we could not precisely calculate the incidence of recurrence and overall survival. Finally, the complications associated with MRND including shoulder syndrome, wound infection, chylous leakage and hemorrhage had not recorded in our medical system. Nevertheless, our study collected data from a single center and used strict inclusion/exclusion criteria that present a reliable and accurate result in the rational management of lateral neck dissection in treatment for N1b PMTC patients.
In conclusion, LNM to lateral level V was relatively low compared with spread to other neck levels in N1b PTMC patients. Routine level V dissection should be considered in PTMC patients who have clinically level V metastasis or with gross ETE and level IV metastasis. Compared with gross ETE, level IV metastasis is superior in predicting level V metastasis.
Acknowledgments
We would like to thank our patients who allowed us to make this study possible.
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 81672885).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-232
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-232
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-232). XL serves as an unpaid associate editor of Gland Surgery from March 2018 to February 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xiangya Hospital Central South University (No.: 201901006) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ullmann TM, Gray KD, Moore MD, et al. Current controversies and future directions in the diagnosis and management of differentiated thyroid cancers. Gland Surg 2018;7:473-86. [Crossref] [PubMed]
- Wang W, Xia F, Meng C, et al. Prediction of permanent hypoparathyroidism by parathyroid hormone and serum calcium 24h after thyroidectomy. Am J Otolaryngol 2018;39:746-50. [Crossref] [PubMed]
- Wang Y, Guan Q, Xiang J, et al. Clinicopathologic features and prognostic factors of diffuse sclerosing variant of papillary thyroid carcinoma: a population-based analysis. Transl Cancer Res 2018;7:695-705. [Crossref]
- Kang JG, Kim YA, Choi JE, et al. Usefulness of 1-year of thyroid stimulating hormone suppression on additional levothyroxine in patients who underwent hemithyroidectomy with papillary thyroid microcarcinoma. Gland Surg 2019;8:636-43. [Crossref] [PubMed]
- Zhou X, Zhou P, Hu Z, et al. Diagnostic Efficiency of Quantitative Contrast-Enhanced Ultrasound Indicators for Discriminating Benign From Malignant Solid Thyroid Nodules. J Ultrasound Med 2018;37:425-37. [Crossref] [PubMed]
- Sugitani I, Ito Y, Miyauchi A, et al. Active Surveillance Versus Immediate Surgery: Questionnaire Survey on the Current Treatment Strategy for Adult Patients with Low-Risk Papillary Thyroid Microcarcinoma in Japan. Thyroid 2019;29:1563-71. [Crossref] [PubMed]
- Lin DZ, Qu N, Shi RL, et al. Risk prediction and clinical model building for lymph node metastasis in papillary thyroid microcarcinoma. Onco Targets Ther 2016;9:5307-16. [Crossref] [PubMed]
- Siddiqui S, White MG, Antic T, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid 2016;26:807-15. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Shaha AR, Tuttle RM. Thyroid cancer staging and genomics. Ann Transl Med 2019;7:S49. [Crossref] [PubMed]
- Kim SK, Park I, Hur N, et al. Should Level V Be Routinely Dissected in N1b Papillary Thyroid Carcinoma? Thyroid 2017;27:253-60. [Crossref] [PubMed]
- Xue S, Wang P, Zhang Q, et al. Routine Lateral Level V Dissection May Not Be Necessary for Papillary Thyroid Microcarcinoma With Lateral Lymph Node Metastasis: A Retrospective Study of 252 Cases. Front Endocrinol (Lausanne) 2019;10:558. [Crossref] [PubMed]
- Battoo AJ, Sheikh ZA, Thankappan K, et al. Level V clearance in neck dissection for papillary thyroid carcinoma: a need for homogeneous studies. Int Arch Otorhinolaryngol 2018;22:449-54. [Crossref] [PubMed]
- Won HR, Chang JW, Kang YE, et al. Optimal extent of lateral neck dissection for well-differentiated thyroid carcinoma with metastatic lateral neck lymph nodes: A systematic review and meta-analysis. Oral Oncology 2018;87:117-25. [Crossref] [PubMed]
- Kang BC, Roh JL, Lee JH, et al. Candidates for limited lateral neck dissection among patients with metastatic papillary thyroid carcinoma. World J Surg 2014;38:863-71. [Crossref] [PubMed]
- Attard A, Paladino NC, Lo Monte AI, et al. Skip metastases to lateral cervical lymph nodes in differentiated thyroid cancer: a systematic review. BMC Surg 2019;18:112. [Crossref] [PubMed]
- Lim YC, Koo BS. Predictive factors of skip metastases to lateral neck compartment leaping central neck compartment in papillary thyroid carcinoma. Oral Oncol 2012;48:262-5. [Crossref] [PubMed]
- Lee JH, Chun YS, Chung YS. Extent of lateral neck dissection for papillary thyroid microcarcinomas. Head Neck 2019;41:1367-71. [Crossref] [PubMed]
- Abraham E, Roshan D, Tran B, et al. The extent of extrathyroidal extension is a key determinant of prognosis in T4a papillary thyroid cancer. J Surg Oncol 2019;120:1016-22. [Crossref] [PubMed]
- Mitchell AL, Gandhi A, Scott-Coombes D, et al. Management of thyroid cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 2016;130:S150-60. [Crossref] [PubMed]
- Kumar S, Burgess C, Moorthy R. The extent of lateral lymph node dissection in differentiated thyroid cancer in the N+ neck. Eur Arch Otorhinolaryngol 2013;270:2947-52. [Crossref] [PubMed]
- Javid M, Graham E, Malinowski J, et al. Dissection of Levels II Through V Is Required for Optimal Outcomes in Patients with Lateral Neck Lymph Node Metastasis from Papillary Thyroid Carcinoma. J Am Coll Surg 2016;222:1066-73. [Crossref] [PubMed]
- Lee YC, Na SY, Park GC, et al. Occult lymph node metastasis and risk of regional recurrence in papillary thyroid cancer after bilateral prophylactic central neck dissection: A multi-institutional study. Surgery 2017;161:465-71. [Crossref] [PubMed]
- Eskander A, Merdad M, Freeman JL, et al. Pattern of spread to the lateral neck in metastatic well-differentiated thyroid cancer: a systematic review and meta-analysis. Thyroid 2013;23:583-92. [Crossref] [PubMed]
- Farrag T, Lin F, Brownlee N, et al. Is routine dissection of level II-B and V-A necessary in patients with papillary thyroid cancer undergoing lateral neck dissection for FNA-confirmed metastases in other levels. World J Surg 2009;33:1680-3. [Crossref] [PubMed]
- Kupferman ME, Weinstock YE, Santillan AA, et al. Predictors of level V metastasis in well-differentiated thyroid cancer. Head Neck 2008;30:1469-74. [Crossref] [PubMed]
- Shim MJ, Roh JL, Gong G, et al. Preoperative detection and predictors of level V lymph node metastasis in patients with papillary thyroid carcinoma. Br J Surg 2013;100:497-503. [Crossref] [PubMed]
- Wang Y, Guan Q, Xiang J. Nomogram for predicting level V lymph node metastases in papillary thyroid carcinoma with clinically lateral lymph node metastases: A large retrospective cohort study of 1037 patients from FDUSCC. Journal of Cancer 2019;10:772-8. [Crossref] [PubMed]


