
Active surveillance for low-risk small papillary thyroid cancer in North American countries: past, present and future (bridging the gap between North American and Asian practices)
The thyroid cancer overdiagnosis epidemic
The incidence of thyroid cancer, especially subcentimetric papillary thyroid carcinoma (i.e., papillary thyroid microcarcinomas or PTMC, pT1a) which represent 50% of all PTCs, has increased in many countries during the past 40 years (1-13). In Canada, thyroid cancer was the most commonly diagnosed cancer (16%) among youth and young adults (aged 15 to 29 years) with 8,200 new cases in 2019 (6,7). Despite the increased incidence of PTC, the mortality rates from thyroid cancer have remained largely stable, indicating possible indolent clinical behavior of the vast majority of thyroid cancers which were identified and treated during that time (8-13). The 5 and 10 years’ survival rates for thyroid cancer in Canada and in USA are reported to be 98% and 97%, respectively, which are the highest of all malignancies (1,4-8). This global phenomenon, which has been referred to as an epidemic of overdiagnosis, is hypothesized to be the result of the increased detection of small PTCs due to evaluations of small thyroid nodules using high‐resolution ultrasound (US), or incidental detection on imaging studies, and fine needle aspiration biopsy (FNAB) (1-14). It is estimated that among women in the USA between 1998 and 2007, 228,000 cases of thyroid cancer (70–80% of cases) were asymptomatic lesions that may have gone undetected during a patient’s lifetime had they not undergone US or other imaging studies (15). Similarly, among women, 90% of cases in South Korea, 70–80% of cases in Italy, France, and Australia, and 50% of cases in Japan likely represented overdiagnosis during that time period (15). Since the prognosis of PTMC is excellent following surgery, some thyroid experts suggest to rename this entity as papillary microtumor (16) or to classify it in the newly recognized borderline tumor category (17), in order to remove the term “cancer” and its negative implications. On the other hand, it is well established that a minor subset of PTMC exhibit true malignant behavior with locoregional and distant metastasis and that there are currently no clinically reliable criteria to identify those patients at presentation (1,18,19). Al-Qurayshi et al. analyzed a total of 30,180 adult patients (25,834 of them as Caucasian) with PTMC and found that PTMC exhibit advanced features (e.g., extrathyroidal extension, lymphovascular invasion, lymph node or distant metastases) in 19% of the patients who had surgery (18). Nilubol and Kebebew analyzed the Surveillance, Epidemiology, and End Results database records of 61,523 thyroid cancer patients between 1988 and 2007, of whom 1,753 (2.8%) died of thyroid carcinoma (19). Of a total of 34,993 patients with T1 (≤2 cm) tumors (62% of all tumors), 253 patients (0.7%) died of thyroid cancer related causes, and among patients with PTMC, approximately 0.5% died of this disease (19). Furthermore, the true potential (natural history) of PTMCs that were treated surgically are unknown. In other words, these PTMCs may have remained indolent, on the other hand the surgery may have been an effective way to avoid progression to a more aggressive entity. Identifying the minor subset of patients with small PTCs which will behave aggressively and thus would benefit from surgical intervention, from those who would benefit from more conservative treatment approaches is a challenge for thyroid specialists (20-23). Many high‐risk features cannot be accurately determined preoperatively and the role of molecular mutations such as BRAF V600E is not clearly established (1,20-23). Currently, in many centers across the world, it is discouraged to perform a biopsy of a thyroid nodule <1 cm in most patients (1,22). For those patients undergoing biopsy and diagnosed with PTMC, the most practical method to deal with the situation is to treat with surgery. Active surveillance (AS), defined as regular monitoring until treatment is required due to disease progression, has been proposed as a valid alternative to patients in this group meeting specific criteria (1,2,22-42). To date, the most comprehensive studies of AS in PTMC were conducted in Japan by the Kuma Hospital group in Kobe, led by Dr. Miyauchi, and the Cancer Institute Hospital in Tokyo, led by Dr. Sugitani (25-28). Their accumulating evidence over the years has shown that AS is a safe and effective alternative to immediate surgical resection for low-risk PTMC in select patients with a low rate of cancer progression during AS, and that surgery performed later when progression is noted is safe and effective (1,25-28,32,41-43). Subsequently, AS has been proposed in other parts of the world as a means to reduce overtreatment and treatment-related complications in patients with low-risk PTC (22,24,29,31,34,35,37-39,41-43). Despite the evidence that AS is a safe and effective alternative to immediate surgical resection in select patients in Japan, the acceptance, feasibility and results of AS in patients with low-risk PTMC in populations other than the Japanese population are still largely unknown. AS is not equally accepted by all physicians around the world because the evidence to support AS in PTMC is still limited, and the applicability of findings from Japanese studies to other populations has been questioned (24). Indeed, cultural differences in terms of the acceptance of AS instead of immediate surgery as well as the natural history and genetics of low-risk PTMC are expected (24,29,31,44,45). Also clinicians and patients have different perceptions of cancer/carcinoma, and until recently the majority of them assumed that cancer will inevitably progress and metastasize, leading to death (14). As a result, many clinicians recommended total thyroidectomy followed by radioactive iodine (RAI) ablation to the patient, and many patients accepted it as shared decision making. This is reflected in the rate of total thyroidectomy which was still high (>80%) for small (<2 cm) localized thyroid carcinomas in 2014 in the USA (11), with approximately 30% of patients with low-risk thyroid carcinoma still receiving RAI in 2015 in the USA (46). More recently, however, the 2015 American Thyroid Association (ATA) thyroid cancer management guidelines have endorsed AS as an alternative approach to immediate surgery in subcentimetric thyroid nodules with highly suspicious US characteristics and in cytologically confirmed very low risk PTC (1). Other associations like The Korean Thyroid Association and Brazilian experts’ consensus have issued similar recommendations (22,47). In contrast, latest clinical guidelines from American Association of Clinical Endocrinologists 2016 (48) and the British Thyroid Association 2014 (49) do not even discuss AS.
The current review intends to summarize the development and the rationale of AS for small thyroid cancers and highlights major differences between North America, with the example of Canada, and Asia, with the example of Japan who pioneered AS.
History of active surveillance for papillary microcarcinoma
In analogy with thyroid cancer, prostate cancer represents another area with a high incidence and low mortality that also came under scrutiny for overdiagnosis but where long-term, randomized prospective clinical trials have already established AS as a first-line treatment approach for properly stratified low-risk patients (2). A recent review published in JAMA Oncology outlined the similarities and differences between thyroid and prostate cancers regarding AS, screening, and diagnosis, and the lessons that endocrinologists and thyroid surgeons could learn from the practice of AS in a selected group of patients (2). AS is generally preferred for low-risk prostate cancer patients older than 70 years due to the morbidity resulting from surgical intervention as well as the lack of survival benefit after treatment. As prostate cancer shows higher disease progression rates, distant metastasis rates, cancer-specific mortality, and lower overall survival than thyroid cancer, AS could be an even more effective and appropriate approach for thyroid cancer than for prostate cancer. However, in contrast to prostate cancer, thyroid cancer patients are typically younger and in the prime of their lives (20–60 years old, and requiring long term follow-up) and thyroid surgery, especially lobectomy, is less morbid than prostate surgery, making them less than ideal candidates (2).
In 1993, Dr. Miyauchi hypothesized that most PTMCs remain small and are harmless to patients (self-limiting cancer). Indeed, PTMCs are frequently detected incidentally (up to 36%) at autopsy of patients who died of unrelated causes, without a history of thyroid malignancy, and in patients who underwent thyroidectomy due to benign or malignant disease of the gland, suggesting that the progression of PTMC to a clinically manifest stage occurs only in a small portion of these tumors (25). Dr. Miyauchi questioned the belief common at that time that PTMCs are an early stage of clinical disease that will automatically progress and should immediately be treated surgically (25). He considered that surgical treatment for all PTMCs might result in more harm than good for the patients and for the health care systems. He considered that delayed (“conversion”) surgery after the appearance of signs of progression such as growth and novel appearance of lymphadenopathy detected on US would still be a timely and effective treatment (25). However, there were no reliable markers predicting the future progression of PTMCs at that time, a situation that continues to this day (see below). As a result, AS was and remains the only method available for discerning PTMCs showing disease progression from others. In 1993, Dr. Miyauchi initiated a clinical study of AS for low-risk PTMC at Kuma Hospital (25). Two years later, in 1995, he was followed by Dr. Sugitani from the Cancer Institute Hospital in Tokyo (25). The Kuma hospital inclusion criteria were: tumor size ≤1 cm and absence of: high-risk location (tumor adjacent to the trachea or recurrent laryngeal nerve), extrathyroidal extension, cervical lymph node involvement, and high-grade malignancy on cytology (25-27). Initially two management options, AS and surgery, were proposed almost equally to the patients. Patients who decided to undergo AS were followed up by US 6 months after the diagnosis of PTMC and at least once per year thereafter to check for signs of progression, such as enlargement or novel appearance of node metastasis (25-27). During AS, surgery was recommended and performed if any signs of progression were detected; otherwise, patients continued AS. Surgery was recommended when tumor size increases by ≥3 mm as compared to the initial size. Depending on patient preference, AS could be continued until tumor size reached 13 mm (25-27). PTMCs with novel appearance of lymph node metastases were strong candidates for surgery. The first report of AS from Kuma Hospital was published in 2003 (25,26), while the Cancer Institute Hospital published their first study in 2010 (28). In their prospective trial, 8% of 1,235 PTMC patients demonstrated tumor enlargement ≥3 mm and 3.8% demonstrated novel lymph node metastases at 10-year follow-up (25-27). Tumor growth and new appearance of lymph node metastases occurred more frequently in patients <40-year-old compared with those >60-year-old (5.9% vs. 2.2% for size increase; 5.3% vs. 0.4% for new nodal metastases, P<0.05) (25-27). The Cancer Institute Hospital reported that 7% of 230 cases had increased in size and 1% had developed apparent lymph node metastasis during mean AS period of 5 years (28). In both studies, for patients with tumor growth or node metastasis during AS, delayed rescue surgery achieved excellent outcomes. There were significantly less unfavorable events (mainly surgery complications) and medical costs in AS group patients. No thyroid cancer related death was reported. Thus, an increasing number of low-risk PTMC patients in these Centers chose AS as their initial management strategy (see below) (33). The Japanese Clinical Guidelines for treatment of thyroid tumor approved AS as the first line of management for low-risk PTMC patients in 2010 (32,43).
Data accumulation on active surveillance (meta-analyses)
As time has progressed, several other research groups from South Korea, USA, Columbia and Brazil have added their results on AS for small PTCs up to 1.5 cm (24,34,35,38,39), and two systematic reviews (meta-analyses) of primary AS management of low-risk PTC were recently published (41,42). In the largest meta-analysis (42), nine publications with 4,156 patients were included: 3,120 from Japan (75%), 688 from South Korea, 291 from USA, and 57 from Colombia. The pooled proportion of tumor growth during AS was 4.4% [95% confidence interval (CI): 3.2–5.8%]. The pooled rate of metastatic spread to cervical nodes was 1.0% (95% CI: 0.7–1.4%). Eight studies assessed incidence of delayed thyroid surgery with pooled proportion of 9.9% (95% CI: 6.4–14.0%). Interestingly, the main indication for surgery was patient preference at 51.9% (95% CI: 44.9–58.9%), probably related to anxiety, rather than disease progression. The pooled proportion of recurrence after delayed thyroid surgery was 1.1% (95% CI: 0.1–3.8%). In this meta-analysis, however, the authors enrolled many overlapped patients. Also, they demonstrated that pooled mortality due to thyroid cancer was 0.03%, which is inaccurate since none of the enrolled patients died of thyroid cancer. The other systematic review and meta-analysis (41) included 6 studies, including 5 from the other meta-analysis (42), and found similar results. Both studies conclude that AS appears to be a safe alternative to surgery for the management of low-risk PTC, without increased risk of recurrence or death (41,42).
Interestingly, while the majority of low-risk PTMCs remain stable in size, tumor size reduction during AS was reported in some series at a frequency ranging from 5–15% (21,41,42). Spontaneous complete or partial regression of PTMCs, akin to other tumors such as seminoma or melanoma, have been described (50). Furthermore, when significant tumor growth does occur, it is highly likely that the tumor will stop growing and may even shrink. In the study by Ito et al., the majority of PTMCs (824 cases) had significantly decreased growth following initial enlargement, which would have traditionally been recommended for conversion surgery (51). This decreased growth rate suggests that continued AS may be considered in select patients but more studies will be needed to define the optimal timing of conversion surgery (51).
It is important to note that the tumors were all <1 cm (pT1a), except in 4 studies (24,34,35,52), since the commonly accepted indication for AS is limited to PTMCs, although AS of larger lesions (up to 2 cm) may be considered provided the tumor is intrathyroidal (1).
In the recent study by a Japanese group (52), of the 392 T1bN0M0 patients, only 61 selected AS over surgery and eventually participated in the trial, while the remaining 331 patients underwent surgery. This suggests that even among a Japanese cohort, the acceptance of AS by patients with pT1b tumors is significantly less than in patients with pT1a (see below). The progression rate did not differ significantly from that of PTMC with a mean observation period of 7.9 years (range, 1–17 years), and delayed rescue surgery was not associated with any deleterious outcomes (52).
A trial from the Memorial Sloan Kettering Cancer Center (MSKCC) included 59 patients with intrathyroidal T1b (<1.5 cm) tumors (35). Among those, two (3.4%) patients showed a tumor diameter increase of >3 mm. This was also not significantly different from their patients with T1a tumors (9/232; 3.9%), but the median follow-up period was only 25 months (range, 6–166 months) (35). The Brazilian experience that was recently published (not included in the meta-analyses), with tumors up to 1.2 cm, reproduced the results observed in other populations, with tumor progression being uncommon despite the limited follow-up and patients (1/70 patients with 30 months follow-up) (24).
These results are encouraging and suggest the safety of AS for patients with small T1bN0M0 PTC in the short-term, but more studies with larger cohorts and long-term follow-up from different centers are required to confirm these data and to define the maximum tumor size appropriate for AS.
Fine needle aspiration biopsy of thyroid nodules. Determining when it is necessary
Overdiagnosis and overtreatment of very low-risk PTC has been an emerging issue not only for patient quality of life, but also for public health management. Given the significant implications each cancer diagnosis has on individuals and the healthcare system, when and how cancers are diagnosed and treated must always be taken into careful consideration. There are significant differences in the practices and culture between Japan and the rest of the world which may explain why AS is still controversial and not as widely used for PTMC in North American countries as opposed to Japan. The Japan Society of Ultrasonics in Medicine and the Japan Association of Breast and Thyroid Sonology recommend observation for solid thyroid nodules with a diameter of <5 mm (43). For solid nodules measuring 5–10 mm, FNAB is advocated only when features highly suggestive of malignancy are present on US (43). At present, this is the only guideline on performing FNAB on thyroid nodules in Japan (25). Therefore, in most Japanese institutions, thyroid nodules measuring ≥5 mm are sampled with FNAB and patients are informed of the diagnosis (e.g., PTMC) (25,32). They consider that receiving a diagnosis of malignancy (PTMC) should encourage patients to visit the hospital for monitoring and increase the acceptance for AS (25,32). Also, without receiving the diagnosis of malignancy, some patients might visit other doctors/hospitals and still undergo FNAB eventually leading to surgery and potential litigation over PTMC “misdiagnosis” (25,32). This strategy appears to work as, according to the experience of the Kuma Hospital, the frequency of AS in PTMC increased from 30% between 1993–1997 to 88% between 2014–2016 (33). It can be argued, however, that patients would be more likely to opt for follow-up/AS rather than surgery if they had a diagnosis of a “small thyroid nodule” for which a biopsy was not performed, as opposed to having an FNAB proven “cancer” (PTMC) that may “open Pandora’s box” and by eliciting fear and anxiety often provokes a strong instinctive, culturally rooted need to proceed with surgery (14). Surgery can provide peace of mind in a subset of patients whose primary concern is to have the “cancer” or “potential danger removed” (14). Counseling such patients not to treat a diagnosed cancer can be difficult. Thus, one strategy that could increase the acceptance of AS is a follow-up in which FNAB is not performed for suspicious nodules ≤1 cm without extrathyroidal extension or apparent lymph node involvement on US. In fact, this is the current recommendation of several associations including the ATA (1,22,24). In contrast to Japan (and possibly a few other countries), in many centers around the world including North America, Europe, UK, Brazil and Australia, it is uncommon practice to biopsy thyroid nodules of <1 cm. In this setting, AS is being done indirectly, as a subset of these nodules harbor a PTMC. In North America, numerous measures have recently been implemented to prevent the overdiagnosis of thyroid cancer, including recommendations to limit unnecessary screening, imaging, and FNAB (1,10,48). The U.S. Preventive Services Task Force has recommended against screening for thyroid cancer in asymptomatic adults using either neck palpation or US in 2017 (53). Clinicians are therefore recommended not to screen for thyroid cancer either with US, neck palpation, or other modalities in asymptomatic patients. Since most thyroid cancers that would be found by screening would be low risk subclinical disease, it is theorized that finding these cancers would be unlikely to change mortality rates, and would lead to potential harms from treatment (53). Nevertheless, it is also plausible that these subclinical thyroid malignancies were treated effectively, avoiding future local and distant spread. In the ATA guidelines from 2009, routine FNAB was only recommended in the following scenarios: (I) family history of PTC; (II) history of external beam radiation exposure as a child; (III) exposure to ionizing radiation in childhood or adolescence; (IV) history of prior hemithyroidectomy with discovery of thyroid cancer; and (V) 18FDG-PET–positive thyroid nodules. In contrast, the latest ATA guidelines from 2015 are even more restrictive and do not recommend FNAB for most thyroid nodules <1 cm (i.e., suspected of PTMC), regardless of clinical or imaging (US or FDG-PET) features. Generally, only nodules ≥1 cm should be biopsied, since they have a greater potential to be clinically significant cancers. On rare occasions, nodules <1 cm may require further evaluation because of clinical symptoms or associated lymphadenopathy. Indeed, nodules <1 cm that lack clinical and US warning signs may lead to future morbidity and mortality, but this is rare. Given the unfavorable cost/benefit considerations, attempts to diagnose and treat all such small thyroid cancers in an effort to prevent rare outcomes may in fact cause more harm than good. To date, there is no evidence regarding the impact of these recommendations, but they should naturally lead to further reduction in the cytologic diagnosis of PTMC.
Similarly, in Brazil it is recommended to perform a FNAB of thyroid nodules ≤1 cm only when the nodule is classified as “very suspicious” (i.e., high suspicion according to ATA (1), high risk according to AACE, TI-RADS 5) and in selected cases (age <40 years, nodule adjacent to the trachea or recurrent laryngeal nerve, multiple suspicious nodules or suspicious lymph nodes, hypercalcitoninemia) (24). Monitoring with US is recommended with a FNAB when the nodule >1 cm (24). With these guidelines, most patients with PTMC are thus already appropriately selected for either close observation or for additional investigations with a FNAB that will often lead to surgery. As a result, in these countries following the ATA or similar guidelines, including Canada, there is only a very limited subset of patients that may actually qualify for AS for a PTMC that was confirmed cytologically, akin to the Japanese population. This is a major difference in patient management and selection between North American and Asian countries (i.e., Japan), but the end results may be similar as eventually more patients will end up being actively monitored/followed-up, with or without a cytologically confirmed PTMC diagnosis, rather than having immediate surgery.
Current guidelines for the management of low-risk thyroid carcinomas
In order to reduce overdiagnosis and overtreatment of small low-risk thyroid carcinomas, the ATA 2015 guidelines stresses the underlying theme that “less is more” (1). Recommendation #12 states that “If a cytology result is diagnostic for primary thyroid malignancy, surgery is generally recommended” (1). They recommend hemithyroidectomy for low-risk, unifocal, intrathyroidal PTMCs with the absence of clinically detectable cervical node metastasis (1). Total thyroidectomy may be opted for high-risk PTMC (locoregional nodal metastases or gross extrathyroidal extension), multifocal PTMC, or to enhance RAI (1). However, an AS management approach can now be considered as an alternative to immediate surgery in patients with very low-risk tumors (e.g., PTMC without clinically evident metastases or local invasion, and no convincing cytologic evidence of aggressive disease) (1). The 2015 ATA guidelines define the candidate patients for AS as follows: very low-risk tumors, high surgical risk (comorbid conditions), relatively short remaining lifespan or concurrent medical/surgical issues that need to be addressed prior to thyroid surgery (1). Importantly, the guidelines do not limit candidates to PTMCs, but merely state that the lesion should be clinically low risk, technically encompassing intrathyroidal PTCs up to 1.5 or 2 cm in size without radiological or clinical nodes (1,54). The KTA has recommended using the same criteria for AS of PTMC (47). The Brazilian experts’ consensus recommends AS rather than immediate surgery as an option in many cases for patients submitted to FNAB whose result of cytology or molecular testing is compatible with PTC (22,24). The American Association of Clinical Endocrinologists 2016 guidelines, however, does not discuss AS per se but merely state that in some elderly patients with incidentally discovered PTMC who are at high surgical risk and have no evidence of extrathyroid spreading, a close clinical and US follow-up may be acceptable (48).
Clinical framework for risk stratification in decision-making for active surveillance for small papillary thyroid carcinomas
Since the latest ATA guidelines from 2015, the selection criteria of patients for an AS management approach have been better defined. The MSKCC, one of the pioneers of AS in North America, recently developed a clinical framework for risk stratification in decision-making for AS in small PTCs in collaboration with Kuma Hospital (37,54). They categorized candidates into three groups (ideal, appropriate, inappropriate) on the basis of various tumor/neck US findings (size and location of the primary tumor, FNAB findings, nodal status), patient characteristics (age, comorbidities, acceptance of AS and compliance with AS protocols), and medical team characteristics (availability of high-quality neck US, experienced multidisciplinary management team) (37,54). This comprehensive approach includes the characteristics of patients and medical teams used in decision making, with the objective of improving the reliability of decision-making and achieving better results. Critical for AS is the presence of significant thyroid US expertise. Thus, large medical centers with high-quality US surveillance capacity for appropriate patient selection and early detection of cancer progression may be more appropriate to adopt AS. Tumor factors and patient factors must also be strongly considered for optimal patient selection. Obviously, individual patient preference, anxiety, and compliance with surveillance protocols and follow-up is essential for a successful AS strategy, in addition to other factors such as age, family history, and pathology (37,54). In the MSKCC trial on AS, however, only 5% of the patients were deemed “ideal candidates”. This fact shows that, in practice, the adoption of this clinical framework may actually not encourage AS.
Additional studies are needed to define important management issues that arise during an AS follow-up approach. This includes the optimal frequency of US during AS, optimal TSH levels, the potential role of serum Thyroglobulin, and specific indications for conversion surgery, including the criteria to define a clinically significant growth of the primary tumor or clinically significant lymph node metastases (1). Tumor enlargement has been regarded as an indicator for surgical intervention during AS. In Japan, Kuma Hospital and the Cancer Institute Hospital have evaluated tumor size increase using the maximal increase in diameter ≥3 mm as a criterion and the MSKCC in the US has also adopted this threshold for selecting patients (25-28,37). Recent studies, however, suggest that determination of the three-dimensional volume is a more sensitive marker of PTMC growth (35,38,39).
Molecular biomarkers for papillary thyroid microcarcinoma
Molecular mutations in the decision making process for AS versus surgery, may play a role in the near future as its use becomes more widespread, especially in North America (1,20,21). PTMCs are a heterogeneous group of malignancies caused by different molecular mutations with different potential for aggressive behavior, that could be further subdivided with the use of molecular testing (20-23). Molecular testing may help to select out those self-limiting PTMCs for AS. It is generally accepted that mutations in well-known thyroid cancer oncogenes, such as BRAF V600E and TERT promoter, especially when combined, predict cancer aggressivity in PTC >1 cm (1,20). Currently, however, there is no data to indicate that any molecular findings, such as BRAF V600E mutation, should impact the suitability of a small PTC for AS (1,22,25,54,55). The extensive Japanese, Korean, and more recent North American experiences have not used molecular markers as inclusion or exclusion criteria (54). When taken in isolation, BRAF V600E has a low PPV, while TERT has a low NPV, for detecting PTMC that will progress and spread outside of the thyroid, and therefore they have a limited and controversial role for guiding patient management at the current time (1,22,25,54,55). Yabuta et al. did not detect a TERT mutation in any of surgical specimens of PTMCs that showed tumor enlargement or novel lymphadenopathy or stable disease, indicating that mutation analyses of these genes on FNAB specimens of PTMCs are unlikely to predict progression of the tumors (55). A recent mutational analysis by Perera et al. revealed that there are no clear mutational differences between indolent PTMCs and those that develop nodal metastases (23). Even though BRAF V600E mutation is present in 24–67% of PTMCs, the overall clinical recurrence rate is quite low, ranging from 1–6% (1,21). However, some studies including a meta-analysis suggest a slightly higher risk of recurrence in PTMCs with BRAF V600E mutations as compared to BRAF V600E wild-type after surgery (20,21). Moreover, aggressive behavior of a given thyroid carcinoma is even more likely when it harbors more than one known oncogenic mutation, and specifically BRAF V600E mutation co-occurring with a TERT promoter, PIK3CA, TP53, or AKT1 mutation (1). Such a combination of mutations is seen in a much smaller fraction of PTCs and is expected to serve as a more specific marker of unfavorable outcomes of PTC. Future studies are expected to establish the impact of molecular testing including BRAF V600E, multiple mutations or other genetic alterations on clinical management of patients with PTMC. Meanwhile, in practice, the Brazilian expert consensus opinion is that in cases in which molecular tests are obtained, the presence of RAS or other RAS-like mutations (e.g., PAX8/PPARG rearrangement) or BRAF V600E or other BRAF V600E-like mutations (e.g., RET/PTC fusions) should not modify the management (AS vs. surgery) (22). This contrasts with the opinion of other authors who suggest that non-surgical long-term AS may not be appropriate for patients with BRAF V600E-positive low-risk PTMC (20,21). Finally, the Brazilian panel currently recommends that cases of PTMC exhibiting “high-risk mutations”, like in the TERT promoter or p53, should not be considered for AS (22).
Active surveillance in Canada and in other Western countries
As discussed above, it is uncommon practice in North American centers to biopsy thyroid nodules that are <1 cm. This may contribute to the lack of uptake of AS in these countries. However, follow-up of patients initially not submitted to FNAB and patients with low-risk PTMC not submitted to surgery (i.e., AS) is essentially the same. According to the ATA 2015 guidelines (recommendation #24), follow-up for nodules that do not meet FNAB criteria should be based upon the nodule’s US pattern (1). For nodules with high suspicion US pattern: repeat US in 6–12 months. For nodules with low to intermediate suspicion US pattern: consider repeat US at 12–24 months. Therefore, biopsy-proven disease is not a requirement for an AS-like management program. This term AS, once called watchful waiting, can also be applied to clinical scenarios where the clinical, imaging, or cytological findings are indeterminate or suggestive of malignant disease [e.g., thyroid nodules with very suspicious US features, or cytology classified as indeterminate (Bethesda III-IV categories) or suspicious for PTC (Bethesda V category)] and in patients with known recurrent and/or metastatic cases. In Japan, AS management schemes are also conducted for many cytologically indeterminate thyroid nodule, especially Bethesda IV, in lieu of proceeding directly to diagnostic lobectomy or expensive molecular testing.
Potential adoption of AS for small low-risk PTCs in Western countries may be increased by changing some management aspects (29,56,57). Although the evidence is still limited, there was no significant difference in AS outcomes with regards to tumor growth, cervical lymph node spread, incidence of delayed surgery, or disease recurrence when comparing studies limited to PTMCs and studies with PTCs up to 15 mm (35,52). Expanding the indications for AS to T1b tumor may increase AS feasibility and acceptance in North America and might lead to further reductions in surgery and its associated complications. Griffin et al. examined the proportion of PTC that would meet the criteria for AS as proposed by the MSKCC and Kuma Hospital, and concluded that increasing the size threshold for AS of PTC to 1.5 cm led to 25% of patients being eligible compared to only 6% with the 1 cm threshold (58). However, this does require further evaluation as long-term follow-up are not yet available.
Patient acceptance of AS is highly linked with the presentation of management options used in discussing low-risk PTC, with a significant patient-physician bias in shared decision making (24). In North America, patient and physician preferences suggest strong resistance to AS: one survey of thyroid surgeons in 2016 found that 100% recommended surgery for PTMC on initial patient consultation, and 99% continued to do so after AS was introduced as an option by the ATA guidelines (59). Despite the substantial benefits gained by opting for AS, few patients are choosing to decline immediate surgery. This has been attributed to a lack of physician knowledge regarding who might be most appropriate for AS and comfort with the safety of this option, as well as lack of patient knowledge that AS is a reasonable treatment option for them. However, in the last 3 years, the frequency of AS acceptance reached 97% among endocrinologists who prefer it more often than surgeons (24). Also, in the recent Brazilian study, after the physician had declared his/her preference for AS to patients, almost all patients followed this option, showing that AS was very well accepted in this population (80% overall) (24). Those recent observations suggest that acceptance by the patient may not seem to be a problem when it is addressed by a physician who is confident that AS is the best option. Informed discussion regarding prognosis and management options increases the acceptance of AS, with patients reporting that the availability of more information may have altered their decision to pursue surgery. Other studies that examine decision-making and acceptability of an AS approach to thyroid cancer in patients, family members, and clinicians are required to better understand how to implement this alternative management approach outside of Japan. In Canada, the frequency with which patients with low-risk PTC would prefer AS or surgery (and the rationale for the choice) is currently unknown (29). To this end, the first Canadian observational prospective study based at the University of Toronto examining whether ~200 patients with low-risk PTC <2 cm would choose AS instead of immediate surgery for management was launched in 2016 (29). The results are not expected to be available before 2026. The inclusion and exclusion criteria for this study are listed in Table 1. Of note, if the 2015 ATA guidelines to perform thyroid FNAB are followed (restricted to nodules ≥1 cm in most patients), it can be expected that the vast majority of enrolled patients will have nodules between 1 and 2 cm as opposed to the typical cohorts of patients who had undergone AS in previous studies (up to 1.5 cm). There are many limitations to this study. An important one is that there is no preliminary data in Canada on the feasibility of recruitment of low-risk thyroid cancer patients in the AS arm. Another one is the inclusion of cases that were diagnosed as Bethesda V (suspicious for PTC), since this is an indeterminate cytologic category and the risk of malignancy associated with this category is only 50–75% or 45–60% when NIFTP is considered as malignant or as nonmalignant, respectively (60). This may overestimate and dilute the true proportion of malignancies in the cohorts, resulting in better outcomes. Furthermore, this relatively low risk of malignancy compared with that in Bethesda VI (94–96%) may reduce the level of anxiety in patients and impact shared decision-making. Interestingly, other centers such as MSKCC and Brazil have also included Bethesda V nodules in the AS trials (24,54).
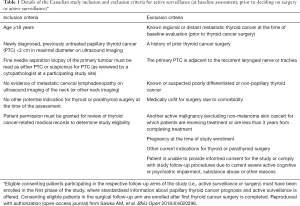
Full table
In addition to Canada, there are a few other ongoing trials on AS in USA, Italy and Korea (Table 2).
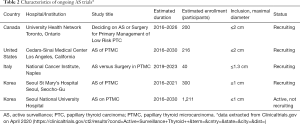
Full table
Estimation of lifetime probability of disease progression on active surveillance
In the Japanese studies, at 10 years of AS, 8% and 3.8% of patients showed tumor enlargement and appearance of nodal metastasis, respectively (25-27). With these data, one might argue that AS is merely postponing surgery. Furthermore, the disease progression rates were significantly lower in older patients (>60) than in younger patients (<40) (25). This fact may be related to the natural selection of indolent longstanding tumors in the elderly group as opposed to a mixture of both indolent and progressive tumors in the younger group which are detected at an earlier period (36). The best estimates of the lifetime probabilities of disease progression with age decade-specific progression rates were 48.9% in patients in their 20s at presentation and 26.7% for those in their 30s; the values then continue to decrease significantly with age at presentation (25,61,62). These estimates still indicate that more than half of patients in their twenties and about 75% of those in their thirties would not require surgery in the first 10 years of AS. Considering the long life-time expectancy and increased chance of annual tumor size increase, young patients have a higher chance of requiring delayed surgery. Therefore, young age should be carefully considered for optimal risk stratification when choosing between AS and immediate surgery (36,37,54). Older patients with PTMC are less likely to progress and are thus even better candidate for AS (33,37,54).
Cost-effectiveness of active surveillance
Medical costs and insurance coverage for AS and immediate surgery vary significantly by country and healthcare system so the data from cost-effectiveness studies on AS need to be taken with caution as they may not be reproducible or useful in another setting/location. In general, the cost and benefit of AS vs immediate surgery for PTMC appear to be in favor of AS at least up to 10 years after diagnosis (25,63-65). However, with the continuing half-yearly US examination and the accumulative possibility of costlier delayed surgery and its related complications over time, AS strategy becomes costlier (64). Therefore, when only cost is considered, AS may be preferable for patients with advanced age or patients with a shortened (≤16 years) life-expectancy, while early surgery may be preferable in younger and healthier patients (with long life-expectancy) which represents the vast majority of patients (64).
Conclusions
Complete standardization of Asian and North American practices including AS will be a challenge since there are many significant differences between populations, cultures, screening methods, preoperative and postoperative diagnosis, surveillance and management between them. Nonetheless, becoming more familiar with the diverse nature of global practices is an important first step. A common treatment for differentiated thyroid tumors including PTMC has yet to find concordant opinions. Currently, no consensus about guidelines for AS versus immediate surgery are available internationally. Centers in Japan have provided evidence that an AS management approach to PTMC is a safe and effective alternative to immediate surgery in properly selected patients. In an era of a thyroid cancer overdiagnosis epidemic, the experience of AS for PTMC in Japan is shifting the paradigms of worldwide treatment. However, robust, long-term longitudinal data from multiple centers are needed to corroborate these findings and to determine the feasibility and acceptance of AS in other countries including Canada. Molecular testing may help to subcategorize PTMCs, which is a heterogeneous group of malignancies, into subgroups that can be selected out for AS. Additional studies are also needed to identify specific markers that would favor surgical resection over AS and to define the optimal timing of surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kennichi Kakudo) for the series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-389). The series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Ho AS, Daskivich TJ, Sacks WL, et al. Parallels Between Low-Risk Prostate Cancer and Thyroid Cancer: A Review. JAMA Oncol 2019;5:556-64. [Crossref] [PubMed]
- La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 2015;136:2187-95. [Crossref] [PubMed]
- The Global Cancer Observatory May, 2019. Available online: https://gco.iarc.fr/today/fact-sheets-populations. (Last accessed on March 22, 2020).
- American Cancer Society: Key Statistics for Thyroid Cancer. Available online: https://www.cancer.org/cancer/thyroid-cancer/about/key-statistics.html. (Last accessed on March 22, 2020).
- Topstad D, Dickinson JA. Thyroid cancer incidence in Canada: A national cancer registry analysis. CMAJ Open 2017;5:E612-6. [Crossref] [PubMed]
- Statistiques canadiennes sur le Cancer 2019. Available online: http://www.cancer.ca/~/media/cancer.ca/CW/publications/Canadian%20Cancer%20Statistics/Canadian-Cancer-Statistics-2019-FR.pdf. (Last accessed on March 22, 2020).
- Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017;317:1338-48. [Crossref] [PubMed]
- Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic"--screening and overdiagnosis. N Engl J Med 2014;371:1765-7. [Crossref] [PubMed]
- Welch HG. Cancer Screening, Overdiagnosis, and Regulatory Capture. JAMA Intern Med 2017;177:915-6. [Crossref] [PubMed]
- Welch HG, Doherty GM. Saving Thyroids - Overtreatment of Small Papillary Cancers. N Engl J Med 2018;379:310-2. [Crossref] [PubMed]
- Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375:614-7. [Crossref] [PubMed]
- Vaccarella S, Dal Maso L, Laversanne M, et al. The impact of diagnostic changes on the rise in thyroid cancer incidence: A population-based study in selected high-resource countries. Thyroid. 2015;25:1127-36. [Crossref] [PubMed]
- Jensen CB, Saucke MC, Francis DO, et al. From Overdiagnosis to Overtreatment of Low-Risk Thyroid Cancer: A Thematic Analysis of Attitudes and Beliefs of Endocrinologists, Surgeons, and Patients. Thyroid 2020;30:696-703. [Crossref] [PubMed]
- Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes 2017;24:332-6. [Crossref] [PubMed]
- Rosai J. Renaming papillary microcarcinoma of the thyroid gland: the Porto proposal. Int J Surg Pathol 2003;11:249-51. [Crossref] [PubMed]
- Kakudo K, Bai Y, Liu Z, et al. Classification of thyroid follicular cell tumors: with special reference to borderline lesions. Endocr J. 2012;59:1-12. [Crossref] [PubMed]
- Al-Qurayshi Z, Nilubol N, Tufano RP, Kandil E. Wolf in Sheep's Clothing: Papillary Thyroid Microcarcinoma in the US. J Am Coll Surg 2020;230:484-91. [Crossref] [PubMed]
- Nilubol N, Kebebew E. Should small papillary thyroid cancer be observed? A population-based study. Cancer 2015;121:1017-24. [Crossref] [PubMed]
- Chen Y, Sadow PM, Suh H, et al. BRAF(V600E) Is Correlated with Recurrence of Papillary Thyroid Microcarcinoma: A Systematic Review, Multi-Institutional Primary Data Analysis, and Meta-Analysis. Thyroid 2016;26:248-55. [Crossref] [PubMed]
- Kim KJ, Kim SG, Tan J, et al. BRAF V600E status may facilitate decision-making on active surveillance of low-risk papillary thyroid microcarcinoma. Eur J Cancer 2020;124:161-9. [Crossref] [PubMed]
- Rosario PW, Ward LS, Graf H, et al. Thyroid nodules ≤ 1 cm and papillary thyroid microcarcinomas: Brazilian experts opinion. Arch Endocrinol Metab 2019;63:456-61. [PubMed]
- Perera D, Ghossein R, Camacho N, et al. Genomic and transcriptomic characterization of papillary microcarcinomas with lateral neck lymph node metastases. J Clin Endocrinol Metab 2019;104:4889-99. [Crossref] [PubMed]
- Rosario PW, Mourão GF, Calsolari MR. Active Surveillance in Adults with Low-Risk Papillary Thyroid Microcarcinomas: A Prospective Study. Horm Metab Res 2019;51:703-8. [Crossref] [PubMed]
- Ito Y, Myauchi A, Oda H. Management of Papillary Microcarcinoma of the Thyroid with A Short Column (Management of Tumors Diagnosed as Follicular Neoplasm on Cytology). In Thyroid FNA Cytology. Kakudo K, eds. Springer 2019.
- Ito Y, Uruno R, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003;13:381-7. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Inoue H, et al. An observation trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010;34:28-35. [Crossref] [PubMed]
- Sugitani I, Toda K, Yamada K, et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 2010;34:1222-31. [Crossref] [PubMed]
- Sawka AM, Ghai S, Tomlinson G, et al. A protocol for a Canadian prospective observational study of decision making on active surveillance or surgery for low-risk papillary thyroid cancer. BMJ Open 2018;8:e020298. [Crossref] [PubMed]
- Hirokawa M, Kudo T, Ota H, et al. Pathological characteristics of low-risk papillary thyroid microcarcinoma with progression during active surveillance. Endocr J 2016;63:805-10. [Crossref] [PubMed]
- Leboulleux S, Tuttle RM, Pacini F, et al. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol 2016;4:933-42. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: A review of active surveillance trials. Eur J Surg Oncol 2018;44:307-15. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kudo T, et al. Trends in the implementation of active surveillance for low-risk papillary thyroid microcarcinomas at Kuma Hospital: gradual increase and heterogeneity in the acceptance of this new management option. Thyroid 2018;28:488-95. [Crossref] [PubMed]
- Sanabria A. Active surveillance in thyroid microcarcinoma in a Latin-American cohort. JAMA Otolaryngol Head Neck Surg 2018;144:947-8. [Crossref] [PubMed]
- Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg 2017;143:1015-20. [Crossref] [PubMed]
- Ho AS, Chen I, Melany M, et al. Evolving management considerations in active surveillance for micropapillary thyroid carcinoma. Curr Opin Endocrinol Diabetes Obes 2018;25:353-9. [Crossref] [PubMed]
- Brito JP, Ito Y, Miyauchi A, et al. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid 2016;26:144-9. [Crossref] [PubMed]
- Kwon H, Oh H-S, Kim M, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center’s experience in Korea. J Clin Endocrinol Metab 2017;102:1917-25. [Crossref] [PubMed]
- Oh HS, Ha J, Kim HI, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: A multi-center cohort study in Korea. Thyroid 2018;28:1587-94. [Crossref] [PubMed]
- Ito Y, Miyauchi A. Active Surveillance as First-Line Management of Papillary Microcarcinoma. Annu Rev Med 2019;70:369-79. [Crossref] [PubMed]
- Cho SJ, Suh CH, Baek JH, et al. Active Surveillance for Small Papillary Thyroid Cancer: A Systematic Review and Meta-Analysis. Thyroid. 2019;29:1399-408. [Crossref] [PubMed]
- Saravana-Bawan B, Bajwa A, Paterson J, et al. Active surveillance of low-risk papillary thyroid cancer: A meta-analysis. Surgery 2020;167:46-55. [Crossref] [PubMed]
- Takami H, Ito Y, Okamoto T, et al. Revisiting the guidelines issued by the Japanese Society of Thyroid Surgeons and Japan Association of Endocrine Surgeons: a gradual move towards consensus between Japanese and western practice in the management of thyroid carcinoma. World J Surg 2014;38:2002-10. [Crossref] [PubMed]
- Nickel B, Brito JP, Barratt A, et al. Clinicians’ views on management and terminology for papillary thyroid microcarcinoma: a qualitative study. Thyroid 2017;27:661-71. [Crossref] [PubMed]
- Nickel B, Brito JP, Moynihan R, et al. Patients’ experiences of diagnosis and management of papillary thyroid microcarcinoma: a qualitative study. BMC Cancer 2018;18:242. [Crossref] [PubMed]
- Park KW, Wu JX, Du L, et al. Decreasing Use of Radioactive Iodine for Low-Risk Thyroid Cancer in California, 1999 to 2015. J Clin Endocrinol Metab 2018;103:1095-101. [Crossref] [PubMed]
- Yi KH. The Revised 2016 Korean Thyroid Association Guidelines for Thyroid Nodules and Cancers: Differences from the 2015 American Thyroid Association Guidelines. Endocrinol Metab (Seoul) 2016;31:373-8. [Crossref] [PubMed]
- Gharib H, Papini E, Garber JR, et al. American association of clinical endocrinologists, american college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules--2016 update. Endocr Pract 2016;22:622-39. [Crossref] [PubMed]
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81:1-122. [Crossref] [PubMed]
- Xu B, Scognamiglio T, Cohen PR, et al. Metastatic thyroid carcinoma without identifiable primary tumor within the thyroid gland: a retrospective study of a rare phenomenon. Hum Pathol 2017;65:133-9. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kudo T, et al. Kinetic analysis of growth activity in enlarging papillary thyroid microcarcinomas. Thyroid 2019;29:1765-73. [Crossref] [PubMed]
- Sakai T, Sugitani I, Ebina A, et al. Active Surveillance for T1bN0M0 Papillary Thyroid Carcinoma. Thyroid 2019;29:59-63. [Crossref] [PubMed]
- U.S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid cancer. U.S. Preventive Services Task Force recommendation statement. JAMA 2017;317:1882-7. [Crossref] [PubMed]
- Lohia S, Hanson M, Tuttle RM, et al. Active surveillance for patients with very low-risk thyroid cancer. Laryngoscope Investigative Otolaryngology 2020;5:175-82. [Crossref] [PubMed]
- Yabuta T, Matsuse M, Hirokawa M, et al. TERT promoter mutations were not found in papillary thyroid microcarcinomas that showed disease progression on active surveillance. Thyroid 2017;27:1206-7. [Crossref] [PubMed]
- Nickel B, Barratt A, McGeechan K, et al. Effect of a change in papillary thyroid cancer terminology on anxiety levels and treatment preferences: a randomized crossover trial. JAMA Otolaryngol Head Neck Surg 2018;144:867-74. [Crossref] [PubMed]
- Alhashemi A, Goldstein DP, Sawka AM. A systematic review of primary active surveillance management of low-risk papillary carcinoma. Curr Opin Oncol 2016;28:11-7. [Crossref] [PubMed]
- Griffin A, Brito JP, Bahl M, et al. Applying criteria of active surveillance to low-risk papillary thyroid cancer over a decade: how many surgeries and complications can be avoided? Thyroid 2017;27:518-23. [Crossref] [PubMed]
- Sacks WL, Zumsteg ZS, Melany ML, et al. Management trends in active surveillance of papillary thyroid microcarcinoma (abstract 98). Presented at: American Thyroid Association 86th Annual Meeting; September 22, 2016; Denver, Colorado.
- Ali SZ, Cibas ES. The Bethesda system for reporting thyroid cytopathology: definitions, criteria and explanatory notes. New York, NY: Springer; 2018.
- Miyauchi A, Kudo T, Ito Y, et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery 2018;163:48-52. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014;24:27-34. [Crossref] [PubMed]
- Oda H, Miyauchi A, Ito Y, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J 2017;64:59-64. [Crossref] [PubMed]
- Lang BH, Wong CK. A cost-effectiveness comparison between early surgery and non-surgical approach for incidental papillary thyroid microcarcinoma. Eur J Endocrinol 2015;173:367-75. [Crossref] [PubMed]
- Venkatesh S, Pasternak JD, Beninato T, et al. Cost-effectiveness of active surveillance versus hemithyroidectomy for micropapillary thyroid cancer. Surgery 2017;161:116-26. [Crossref] [PubMed]


