
Transperitoneal versus extraperitoneal robot-assisted laparoscopic radical prostatectomy on postoperative hepatic and renal function
Introduction
Radical prostatectomy can be performed by traditional open and minimally invasive approaches, such as laparoscopic or robotic-assisted. In recent years, there has been increased adoption, and robotic-assisted laparoscopic radical prostatectomy (RALP) is widely used as a prior therapeutic procedure for prostate cancer patients. Compared with traditional open surgery, it has significant benefits, including reduced blood loss, a shorter hospital stay, and reduced postoperative pain (1,2). But there are not sufficient research about the superiority of any surgical approach in terms of oncological outcomes. It is necessary to keep the patient in the extreme low head and high foot position (steep Trendelenburg position) with artificial pneumoperitoneum during the RALP to optimize the surgical field and operation. The artificial pneumoperitoneum is always associated with the increase of intraperitoneal pressure, which affects venous return, systemic vascular resistance, and cardiac function (3). At the beginning of pneumoperitoneum, the volume of circulating blood increases due to the autotransfusion of mixed blood from visceral circulation. However, with the further increase of intraperitoneal pressure, the inferior vena cava is compressed, and the venous return is reduced as well as the cardiac output. Also, the absorption of pneumoperitoneum gas (mostly carbon dioxide) leads to hypercapnia and a simultaneous decrease in blood pH. The increase in intraperitoneal pressure caused by artificial pneumoperitoneum also leads to the displacement of the diaphragm to the cephalic side of the patient, aggravated by the extreme head, low foot, high position and leads to the decrease of lung compliance and functional residual capacity. These factors eventually result in airway collapse, atelectasis, mismatch of ventilation-perfusion, and finally, a decrease in arterial oxygenation (4), which may, in turn, lead to a series of cardiopulmonary complications.
The decrease of venous reflux caused by high pneumoperitoneum pressure may not only result in a compromised cardiopulmonary function but also visceral function, including liver and kidney, through decreased perfusion. The increased pneumoperitoneum pressure and operation time resulted in ischemia and anoxia of visceral organs. The withdraw of pneumoperitoneum, which leads to re-perfusion injury, may also aggravate damage to hepatocytes (5). Several intraoperative ventilation strategies, as well as lower pneumoperitoneum pressure, were used to improve the oxygenation of patients and reduce the side effects brought by the extreme head-low-foot-high position (6,7). The procedure was improved to ameliorate further the side effects caused by the extreme position and abdominal pressure in RALP. Compared with the transperitoneal approach, the extraperitoneal approach results in less pressure on the abdominal wall, a smaller internal incision with less intestinal irritation. These factors lead to a faster postoperative intestinal recovery. Besides, the patient’s angle of retroversion is 10–15°, which is less than the 30° of the trans-abdominal approach. However, due to the more surgical field TP- RALP can offer, it remains the first choice at present (8,9).
This retrospective research was set out to evaluate the short-term outcome of patients with RALP under two approaches and to help the surgical team choose the appropriate approaches for vulnerable patients, especially those with hepatic or renal insufficiency. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-533).
Methods
Our study is a retrospective, single-center, controlled clinical study. The data of 176 prostate cancer patients who were treated at the urology department of Zhongshan Hospital affiliated to Fudan University from 2015 to 2019 by an identical surgery team were collected. According to the surgery, the effects of different approaches of RALP on the function of visceral organs (liver and renal) were assessed by pre- and postoperative hepatic and renal function tests. The secondary endpoints include intraoperative bleeding volume, postoperative hospital stay, postoperative complications (lymphatic leakage, bleeding, and infection), and short-term postoperative follow-up index [prostate-specific antigen (PSA)]. The entire process is described in the diagram below (Figure 1). The study was approved by The Ethics Committee of the Zhongshan hospital, Fudan University (No. B2019-199R) and written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki.
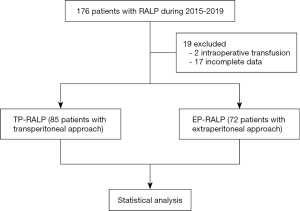
Inclusion criteria
The patients that we included in our study met the following criteria: voluntary participation, confirmed diagnosis of prostate cancer, robot-assisted laparoscopic radical prostatectomy, 18 to 80 years old, the three months follow-up of the surgery had been completed at least, and American Society of Anesthesiologists (ASA) physical status I or II.
Exclusion criteria
The exclusions were as follows: a turn to open surgery during operation; blood transfusion during the operation; serious complications occurring during and after the operation; those who refused to be involved or did not complete follow-up; have the history of hernia or intestinal obstruction; patients with hepatic and renal diseases are excluded.
Surgical procedure
Extraperitoneal approach
A vertical 1.2 cm incision 3 cm inferior to the umbilicus was used to approach the extraperitoneal space. An incision was made into the anterior rectus sheath before the rectus muscle was split. The extraperitoneal space was opened by the insertion of the index finger in a caudal direction. Using an extraperitoneal balloon dilator was space was created before the introduction of a 12 mm trocar. Following an inspection of the extraperitoneal cavity, the first 8-mm robot trocar was placed on the right side under vision, about four fingers laterally and 2 cm lower to the supra-umbilical incision. A second 8-mm robot trocar was placed on the left side, in a similar position as the first. The placement of a third 8-mm robot trocar for the fourth robotic arm was at 1.5 cm, cranially to the left anterior iliac crest under vision. Finally, a 12 mm assistant trocar was placed under vision on the patient’s right side (8).
Operative technique
The steps of the operation included: incisions to the endopelvic fascia and separation of puboprostatic ligaments; paraurethral dissection of the bladder neck; the anterior opening of the bladder neck; using the fourth arm to catch the catheter, allowing for the prostate to be elevated; dissection of the posterior bladder neck; dissection of the vas and seminal vesicles; the opening of the Denonvillers fascia and separation of the neurovascular bundles; deep venous complex ligation free; apical dissection and disconnection of the prostate from the urethra; and ureterovesical anastomosis. Most of the time, pelvic lymph node dissection (PLND) was conducted before the ureterovesical anastomosis. A drain was placed in the pelvic cavity, and the extraction of the specimen was conducted via the middle 12 mm port incision.
The transperitoneal approach
The patient was placed in the Trendelenburg position under general anesthesia with their legs apart. A vertical incision was made 2 cm above the umbilicus, and another vertical 1 cm incision was made to the anterior rectus sheet. A veress needle was used to induce pneumoperitoneum before the introduction of a 12-mm trocar. Following an inspection of the abdominal cavity, the first 8-mm robot trocar was placed under vision on the right side, about four fingers laterally to the supra-umbilical incision on the line joining the sub-umbilical incision and the right anterior iliac crest. A second 8-mm robot trocar was placed on the left side in a similar position to the first. A third robot trocar measuring 8-mm was placed 2 cm cranially to the left anterior iliac crest for the fourth robotic arm. A 12 mm trocar was placed to aid on the patient’s right side. The robot was docked, the dissection of the pelvic peritoneal had taken place, and the extraperitoneal space was created. The rest of the procedure was carried out in a manner, as previously described (9).
Data collection
Our study was a retrospective analysis. Demographic characteristics including age, race, ASA, physical status, and disease history were recorded. Surgical and intraoperative details, including estimated blood loss and length of operation as well as postoperative hospital stay, postoperative complications (lymphatic leakage, bleeding, and infection), pre- and postoperative PSA, were also recorded. The first postoperative PSA was done three months after surgery. The laboratory tests of renal and hepatic function were respectively collected pre-operatively and within one week after the procedures. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), combined bilirubin (CB), total protein (TP), albumin (ALB), globulin (GLO), urea, serum creatinine (Scr), estimated glomerular filtration rate (eGFR), and creatinine clearance (CCR) are used to evaluate the hepatic and renal function. Changing percentages of all data were calculated [(last value − initial value)/initial value ×100%].
Statistical analysis
The SPSS 22.0 statistical software was used for statistical processing. The data were all expressed as the mean ± standard deviation or count with percentage. The comparison between groups was conducted by Student’s t-test or a chi-square test. The normality test was conducted before these tests. Differences were statistically significant when P<0.05.
Results
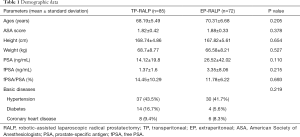
An identical surgery team completed all operations. Tables 1 and 2 show the general preoperative information of the two groups. No statistical difference of age, height, weight, PSA, or free prostate-specific antigen (fPSA) existed between the two groups, while the postoperative hospital stay and the length of operation in the TP-RALP group were significantly higher than those in the EP-RALP group (P<0.05). The estimated blood loss was comparable between the groups.
Full table

Full table
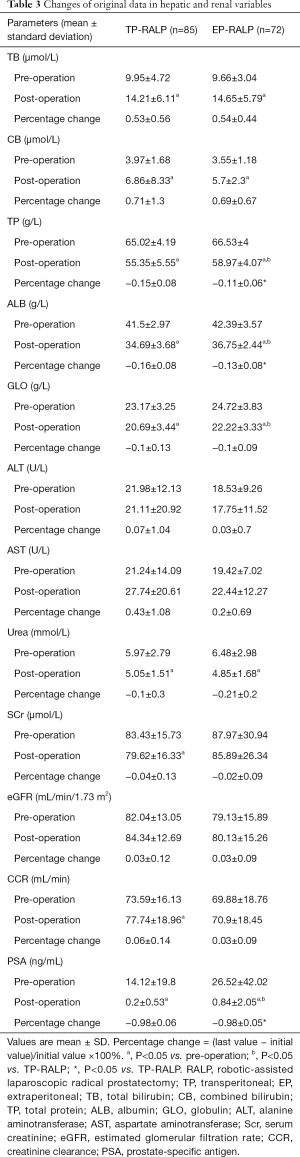
Pre- and postoperative hepatic and renal function in the two groups are shown in Table 3. After the operation, TB and CB were significantly increased, while the total protein, albumin, and globulin were significantly reduced compared with pre-operation (P<0.05) in both TP-RALP and EP-RALP groups. Postoperative levels of urea were significantly reduced (P<0.05) in both groups with no change of eGFR and CCR.
Full table
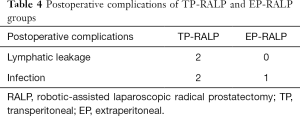
The preoperative hepatic and renal functions are comparable in two groups. The TP, ALB, and GLO in the EP-RALP group were reduced to a less extent than those in the TP-RALP group postoperatively (P<0.05). In terms of postoperative complications, there were two cases with lymphatic leakage and two with infection in the TP-RALP group while 1 case of infection in the EP-RALP group (Table 4). All patients with complications recovered after conservative treatment. Postoperatively, PSA was significantly decreased in both groups (P<0.05).
Full table
Discussion
In this retrospective study, we observed that the transperitoneal and extraperitoneal approaches of RALP were comparable in the short-term outcome. Meanwhile, the hepatic function was affected to a greater extent in the transperitoneal approach than the extraperitoneal approach.
Since the introduction of robot-assisted laparoscopic radical prostatectomy in 2000, it has almost completed replaced laparoscopy for radical prostatectomy in robotic machines affordable areas (10). Compared with open surgery, RALP showed reduced blood loss, early hospital discharge, and early return to everyday life with the equivalent outcome. Also, with the slighter Trendelenburg position and intact peritoneum, the extraperitoneal approach is ideally suited under certain situations like being obese and having a prior history of abdominal surgery (8).
Using low-pressure pneumoperitoneum does not appear to have clinical advantages as compared to standard pressure on cardiac and pulmonary function with proper perioperative measures. As to liver and kidney, low intraabdominal pressure is associated with less injury (11). In the extraperitoneal approach, less pressure exerted on the abdominal wall by the mechanical arm (12). So, our study focused on the liver and renal function only when comparing the outcome of these two approaches. The normal portal vein pressure ranges from 7 to 10 mmHg. A level of intraperitoneal pressure that exceeds this range reduces the portal vein blood flow and could result in hepatic ischemia (13-15). Animal research confirmed that 15 mmHg carbon dioxide pneumoperitoneum caused more substantial hepatic injury (16). In the human study, the plasma disappearance rate (PDR) of indocyanine green (ICG), a compound marker for hepatosplanchnic perfusion, but not classic liver laboratory parameters, was positively correlated to intraabdominal pressure (17). In this retrospective study, with only the availability of laboratory tests of hepatic function, the results showed that under the same insufflation pressure (10 mmHg), some main parameters of hepatic function were changed in the postoperative, period which means pneumoperitoneum did affect the liver function. The hepatic perfusion has a unique self-regulation mechanism, called the hepatic arterial buffer response (HABR). Under physiological and pathophysiological conditions, the change in portal vein blood flow can be offset by a change in hepatic artery flow, to maintain the total hepatic blood flow and ensure the liver has sufficient oxygen supply. However, during carbon dioxide pneumoperitoneum, hepatic artery blood flow cannot compensate for the decrease in portal vein blood flow. One possible explanation was that the absorption of carbon dioxide through the peritoneum could lead to adrenergic activity and hypercapnia leading to acidosis, which was known to affect the regulation of hepatic perfusion (13,18). Further analysis showed that TP and ALB were decreased to a greater extent in the transperitoneal approach than that in the extraperitoneal approach. Considering the same perioperative treatment in both groups, the difference may due to a longer operation in the TP-RALP group which was associated with operative trauma and increased catabolism (19-21).
Our laboratory test of renal function showed that the eGFR and CCR did not affect these two approaches of RALP while the Urea and Scr were slightly decreased postoperatively. A retrospective study carried by other centers showed a similar result. In the normal preoperative CCR group, RARP with an IAP of 15–20 mmHg does not induce renal dysfunction postoperatively (22). Theoretically, an increase in intraperitoneal pressure leads to the compression of renal vessels and parenchyma (23). A decrease in renal perfusion can cause the activation of the renin-aldosterone system and reduce renal blood flow. In the RALP procedure, pneumoperitoneum and steep Trendelenburg position decrease renal blood flow and urine output by increasing intra-abdominal pressure (24). Compared with open retropubic radical prostatectomy (RRP), RALP significantly affected the eGFR, urea, and creatinine levels (25). We did not observe the same decreased renal function in our study. This difference may due to a lower insufflation pressure in our study (10 mmHg) compared to theirs (12–15 mmHg). Importantly, the observed changes in renal function and perfusion may reversible and may also have a significant impact on patients with pre-existing compromised renal function, but minor importance in healthy people (26).
PSA in both groups reduced statistically after surgery, which means the surgical outcome of these two approaches is comparable. Like other studies (14,27), the length of postoperative hospital stay and operation were significantly lower in EP-RALP than that in the TP-RALP.
The limitations of this study are that as a retrospective study, the individual IAP was absent, and only the route laboratory tests of hepatic and renal function were available. More well-designed randomized studies are needed to evaluate further the difference and indications of these two approaches of RALP. Since the extraperitoneal pneumoperitoneum has been gradually started in our hospital since 2018, the follow-up time of most patients is relatively short. For comparison between the two groups, an appropriate length of follow-up time was set. If possible, the follow-up of this study will continue to get more convincing results.
Both transperitoneal and extraperitoneal approaches for undergoing a RALP procedure slightly affect hepatic and renal function to similar extents. Due to a shorter length of operation, the decrease of TP, ALB, and GLO in the EP-RALP group was significantly lower than that in the TP-RALP group. Our results suggest that when it comes to vulnerable patients, including those with preoperative malnutrition or renal and hepatic dysfunction, a decision should be made with greater caution for the surgical approaches.
Acknowledgments
We are thankful to Dr. Shuai Jang and Zhongshan Hospital, Fudan University, for the support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-533
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-533
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-533). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by The Ethics Committee of the Zhongshan Hospital, Fudan University (No. B2019-199R) and written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs. open radical prostatectomy. JAMA 2009;302:1557-64. [Crossref] [PubMed]
- Dommer L, Birzele JA, Ahmadi K, et al. Lower urinary tract symptoms (LUTS) before and after robotic-assisted laparoscopic prostatectomy: does improvement of LUTS mitigate worsened incontinence after robotic prostatectomy? Transl Androl Urol 2019;8:320-8. [Crossref] [PubMed]
- Kakde AS, Wagh HD. An observational study: Effects of tenting of the abdominal wall on peak airway pressure in robotic radical prostatectomy surgery. Saudi J Anaesth 2017;11:279-82. [Crossref] [PubMed]
- Means LJ, Green MC, Bilal R. Anesthesia for minimally invasive surgery. Semin Pediatr Surg 2004;13:181-7. [Crossref] [PubMed]
- Lee JY, Choi SH. Results of hepatic and renal function tests to different CO2 pneumoperitoneum conditions: An experimental capnoperitoneum study in dogs. Res Vet Sci 2015;101:1-5. [Crossref] [PubMed]
- Haliloglu M, Bilgili B, Ozdemir M, et al. Low tidal volume positive end-expiratory pressure versus high tidal volume zero-positive end-expiratory pressure and postoperative pulmonary functions in robot-Assisted laparoscopic radical prostatectomy. Med Princ Pract 2017;26:573-8. [Crossref] [PubMed]
- Lee HJ, Kim KS, Jeong JS, et al. Optimal positive end-expiratory pressure during robot-assisted laparoscopic radical prostatectomy. Korean J Anesthesiol 2013;65:244-50. [Crossref] [PubMed]
- Semerjian A, Pavlovich CP. Extraperitoneal Robot-Assisted Radical Prostatectomy: Indications. Technique and Outcomes 2017;1-7.
- Hoznek A, Antiphon P, Borkowski T, et al. Assessment of surgical technique and perioperative morbidity associated with extraperitoneal versus transperitoneal laparoscopic radical prostatectomy. Urology 2003;61:617-22. [Crossref] [PubMed]
- Costello AJ. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat Rev Urol 2020;17:177-88. [Crossref] [PubMed]
- Gainsburg DM, Wax D, Reich DL, et al. Intraoperative Management of Robotic-Assisted Versus Open Radical Prostatectomy. JSLS 2010;14:1-5. [Crossref] [PubMed]
- Menon M, Tewari A, Baize B, et al. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: The Vattikuti Urology Institute experience. Urology 2002;60:864-8. [Crossref] [PubMed]
- Richter S, Olinger A, Hildebrandt U, et al. Loss of physiologic hepatic blood flow control (“hepatic arterial buffer response”) during CO2-pneumoperitoneum in the rat. Anesth Analg 2001;93:872-7. [Crossref] [PubMed]
- Atug F, Castle EP, Woods M, et al. Transperitoneal versus extraperitoneal robotic-assisted radical prostatectomy: is one better than the other? Urology 2006;68:1077-81. [Crossref] [PubMed]
- Singal R, Singal RP, Sandhu K, et al. Evaluation and comparison of postoperative levels of serum bilirubin, serum transaminases and alkaline phosphatase in laparoscopic cholecystectomy versus open cholecystectomy. J Gastrointest Oncol 2015;6:479-86. [PubMed]
- Li J, Liu YH, Ye ZY, et al. Two clinically relevant pressures of carbon dioxide pneumoperitoneum cause hepatic injury in a rabbit model. World J Gastroenterol 2011;17:3652-8. [Crossref] [PubMed]
- Kovac N, Peric M. Liver function assessment by indocyanine green plasma disappearance rate in patients with intra-abdominal hypertension after “non-hepatic” abdominal surgery. Curr Med Res Opin 2018;34:1741-6. [Crossref] [PubMed]
- Sánchez-Etayo G, Borrat X, Escobar B, et al. Effect of intra-abdominal pressure on hepatic microcirculation: Implications of the endothelin-1 receptor. J Dig Dis 2012;13:478-85. [Crossref] [PubMed]
- Hübner M, Mantziari S, Demartines N, et al. Postoperative Albumin Drop Is a Marker for Surgical Stress and a Predictor for Clinical Outcome: A Pilot Study. Gastroenterol Res Pract 2016;2016:8743187. [Crossref] [PubMed]
- Ryan AM, Hearty A, Prichard RS, et al. Association of Hypoalbuminemia on the First Postoperative Day and Complications Following Esophagectomy. J Gastrointest Surg 2007;11:1355-60. [Crossref] [PubMed]
- Inagaki E, Farber A, Eslami MH, et al. Preoperative hypoalbuminemia is associated with poor clinical outcomes after open and endovascular abdominal aortic aneurysm repair. J Vasc Surg 2017;66:53-63.e1. [Crossref] [PubMed]
- Ahn JH, Lim CH, Chung HI, et al. Postoperative renal function in patients is unaltered after robotic-assisted radical prostatectomy. Korean J Anesthesiol 2011;60:192-7. [Crossref] [PubMed]
- Özdemir-van Brunschot DMD, van Laarhoven KCJHM, Scheffer GJ, et al. What is the evidence for the use of low-pressure pneumoperitoneum? A systematic review. Surg Endosc 2016;30:2049-65. [Crossref] [PubMed]
- Sassa N, Hattori R, Yamamoto T, et al. Direct Visualization of Renal Hemodynamics Affected by. Urology 2009;73:311-5. [Crossref] [PubMed]
- Ergin G, Doluoglu OG, Kıraç M, et al. Comparison of renal function after robot - assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy. Int Braz J Urol 2019;45:83-8. [Crossref] [PubMed]
- Wever KE, Bruintjes MHD, Warlé MC, et al. Renal Perfusion and Function during Pneumoperitoneum: A Systematic Review and Meta-Analysis of Animal Studies. PLoS One 2016;11:e0163419. [Crossref] [PubMed]
- Jacobs BL, Montgomery JS, Dunn RL, et al. A Comparison of Extraperitoneal and Intraperitoneal Approaches for Robotic Prostatectomy. Surg Innov 2012;19:268-74. [Crossref] [PubMed]


