Role of ultrasound in the detection of recurrent ovarian cancer: a review of the literature
Background
Ovarian cancer (OC) is the leading cause of death among gynecological cancers, and the fifth leading cause of death in women among all cancers (1). Despite improvements in technology and the accuracy of radiological and laboratory diagnostic tests, around 60% of OC is actually diagnosed at an advanced stage, which therefore remains the main prognostic factor (1).
The complete surgical removal of the disease, followed by platinum based chemotherapy, has still the greatest impact on survival for advanced OC (2,3), while a fertility sparing surgery (FSS) appears to be safe just in patients with low-grade stage IA (serous, endometrioid or mucinous expansile subtype) (4,5), and acceptable for stage IC1 tumors, where about 50% of recurrences are located in the remaining ovary and therefore suitable for subsequent surgery (6).
The 5-year survival rates have modestly changed for decades, and mostly depend on the stage of the disease at diagnosis, reaching 70–80% in early stage ovarian cancer (ESOC) and dropping down to 20–25% in advanced stage disease (7).
Furthermore, despite promising findings on new targeted therapy regimens, recurrence rates remained high, ranging between 25% and 80% based on the initial stage of disease (8).
The main difficulty in the management of the recurrent disease lies in its inherent chemo-resistance potentially due to the selection of immunoedited and drug-resistant cells during first line chemotherapy (9,10).
Diagnosis of recurrent ovarian cancer (ROC) is still a challenging issue as symptoms are usually unspecific and scar tissue and fibrosis, resulting from surgery and chemo/radiotherapy, can mimic tumor recurrence.
To date, no evidence supports a standard follow-up regimen. NCCN guidelines recommend scheduled clinical visits, radiological examination (chest/abdominal/pelvic CT, MRI, PET/CT, or PET) when clinically indicated and dosage of CA-125 if initially elevated (11).
Among radiological exams, CT scan represents the most adopted exam during follow up, with a sensitivity of 58–84% and a specificity between 60–100%. Its biggest weakness is in the identification of peritoneal, mesenteric or intestinal wall lesions of less than 5 mm, which could remain undetected (12).
PET-CT has indeed the highest sensitivity and specificity (of 45–100% and 40–100% respectively) (13) and it is mainly used in patients with increased CA-125 and negative CT scan.
Several follow up strategies are possible for OC, but all should be tailored to both the patient’s and tumor’s characteristics and should focus on the early detection of recurrent lesions. An earlier recurrence detection could create the possibility of different management strategies, such as the chance to perform secondary cytoreductive surgery in platinum sensitive ROC (14-16) eventually associated with HIPEC (17-20). As a matter of fact, accumulating evidence suggests that the management of ROC should be tailored on performance status and comorbidities. In this scenario, a timely diagnosis of relapses could allow to reduce the percentage of under-treatment in the elderly population (21,22).
Moreover an early detection of the recurrence, especially in the subset of fragile or elderly cancer patients, could help the surgeon to offer, if technically feasible, a minimally invasive approach (23-25) with significant benefits in terms of postoperative complications and quality of life (26,27).
While the role of ultrasound (US) is well defined in primary diagnosis of OC and potentially useful to detect endometriosis-associated OC (28,29), it is still controversial during follow-up of surgically treated OC (30).
Over the last decade, there has been a massive technology development which led to a dramatic improvement in US imaging quality.
US is certainly a cost-effective exam and its non-invasiveness makes it easy to offer.
It is also a valuable procedure for monitoring patients treated with FSS and, furthermore, it is an ideal technique to guide Tru-Cut biopsy of suspicious pelvic lesions (31).
Methods
We performed a review of the English literature present in PubMed and SCOPUS, regarding the use of US in ROC.
The articles’ search was performed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (32,33).
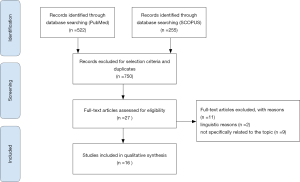
The terms “ultrasound and recurrent ovarian cancer” and “intraoperative ultrasound and recurrent ovarian cancer” were used to search in the above-mentioned databases. A search of the references of both potentially relevant articles and articles qualifying for inclusion was also performed. No publication period restrictions were adopted. The search was concluded in February 2020 (Figure 1).
A narrative description of the findings, structured around the accuracy of pelvic and intraoperative US in ROC, has been carried out.
No statistical analysis or meta-analysis has been performed.
After a crossmatch research, 777 articles were screened. The number of full text articles assessed for eligibility was 27. Exclusion criteria were studies not in English language and non-pertinence with the present topic.
Finally, 16 articles have been considered suitable for analysis (Tables 1,2).
Full table
Full table
Results
Based on the results found in the literature, US was mainly adopted in 3 different settings:
❖ The detection of recurrent disease after debulking surgery (Table 1);
❖ The detection of recurrent disease after FSS (Table 2);
❖ As an intraoperative tool for localization of OC recurrence.
The role of US in the detection of recurrent disease after debulking surgery
Precise determination of tumor dimensions and localization is essential for a rational therapy of ROC. As a matter of fact, US can detect pelvic relapses even if small in size, whereas its major limit is the identification of upper abdominal lesions. Trans-vaginal (TV) and trans-rectal (TR) US probes allow a detailed visualization of the pelvic structures, while trans-abdominal (TA) probes rely on lower frequency probes and the image resolution mostly depends on the dimensions of the lesions and the patient’s habitus.
OC recurrences are mainly described in US as hypoechoic single solid lesions with irregular margins, a moderate to rich vascularization at color Doppler (CD) and are usually associated with ascites or pelvic free-fluid (34-36).
Secondary lesions are usually smaller than the primary tumor, with an average size of 2 to 4 cm whereas the primary tumor is typically larger (8–12 cm for serous OC, 18 to 20 cm for mucinous cancer) (34,36).
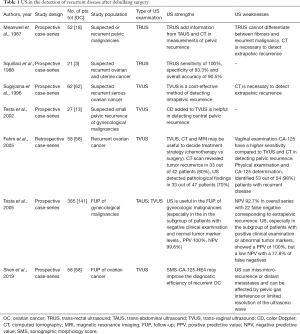
The use of TR-US was first described in 1987 by Meanwell et al. (37) for the early detection of recurrent pelvic malignancies. They found that TR-US had a high level of agreement compared with the CT scan for lesions of at least 1.2 cm. Furthermore, TR-US could provide precious and complementary information concerning the relapse’s localization in the pelvic side walls, useful information about the anterior and posterior pelvic compartments, as well as for the central and presacral region. Similar results have been achieved by Squillaci et al. in 1988 (38) who demonstrated an overall accuracy for TR-US of 90.5% with a sensitivity of 100% and specificity of 83.3%.
Studies that have followed, mainly confirm this data also for TV-US. In 1996, Sugiyama et al. firstly showed how TVUS was a cost-effective method of detecting interpelvic recurrence while CT scan was still considered necessary to detect extrapelvic lesions (39). Testa et al. concluded in 2005 (35), with a prospective multicentric study performed on 385 patients, that TV-US might play a determinant role during follow up of OC, in particular by detecting central pelvic lesions in asymptomatic patients with negative tumor markers [US examination in the subgroup of patients with negative clinical examination and normal tumor marker levels show a positive predictive value (PPV) of 100% and a negative predictive value (NPV) of 99.6%, while in the group of patients with positive clinical examination or abnormal tumor markers, the US analysis showed a PPV of 100% but a low NPV, with 77.8% of false negatives].
Furthermore, the detailed US characterization in terms of location and features of the lesion led to a correct and complete surgical resection, achieving no residual tumor (RT =0) in more than 90% of the recurrences. On the other hand, in symptomatic patients or patients with increased tumoral markers, US failed to visualize retroperitoneal or peritoneal abdominal extrapelvic recurrences while CT scan and MRI remain the milestone for the detection of such OC recurrences.
Specifically, US examination failed to identify 22 cases of recurrences in the overall series of 385 patients, with 21 of these false negative clustered in the subgroup of patients with positive clinical examination or positive tumor marker, and only 1 case in the other group.
In contrast with the study reported above, Fehm et al. described in 2005 (40) the non-superiority of imaging technique (TV-US and CT scan) in the detection of pelvic OC recurrence when compared with the serum dosage of CA-125 and vaginal examination. Indeed, CT scan revealed tumor recurrence in 33 out of 42 patients (79%), US detected pathological findings in 33 out of 47 patients (70%), while follow-up based on physical examination and CA-125 determination, identified 53 out of 54 (98%) patients with recurrent disease.
The main challenge in the correct US identification of pelvic recurrences is to discriminate whether the suspected lesion is a real malignant secondary neoformation or a fibrotic reaction due to previous treatments.
Trying to answer and clarify this issue, Testa et al. in 2002 (34) reported the CD characteristics of recurrent central pelvic lesions, identifying specific criteria useful to correctly discriminate between malignant versus benign lesions. Malignant lesions were characterized by a statistically proven higher velocity flow, lower resistance index and color score of 3 when compared with benign lesions (34).
The role of TA US has not been well investigated. False negative results are predominantly due to lack of specificity and sensibility of the technique in the detection of enlarged retroperitoneal lymph-nodes or thickening of the peritoneum, thus confirming the superiority of CT scan and MRI in the recognition of this type of lesion.
Nevertheless, splenic OC metastases detected by TA US have been described by La Fianza as hypoechoic round irregular lesions, not vascularized at CD (41).
More recently, an effort has been led from Shen et al. in 2019 (36) to overcome the limit of US in the detection of such extra-pelvic or diffuse peritoneal recurrences. They suggested a combination of US features with the rising curve of CA-125 and HE4 in order to potentially detect these recurrences typically misdiagnosed at US. They described recurrent lesions as hypoechoic mass of 3±1.4 cm with peripheral color flow signals and associated pelvic peritoneal free fluid. Papillary echoes have not been described frequently.
In addition to the emerging role of US in this field, TV-US has also been used as a guide for pelvic biopsies in patients with suspected recurrence, not suitable for surgery. In 2008, Fischerova et al. (42) initially described the US Tru-Cut guided biopsy as an added tool to the US diagnostic technique in recurrent gynecological cancer. Besides, it can be offered in an outpatient setting with a reported complication rate of less than 1%, an adequate sampling in 95% of cases and an accurate diagnosis achieved in 98% of cases.
Similarly, Zikan et al. confirmed the safety and feasibility of this procedure by analyzing the results from 195 Tru-Cut biopsies performed either transvaginally or transabdominally, and showing the achievement of an adequate sample in 178 (91.3%) cases with only 2 reported post-operative complication (1%) (43).
Finally, Mascilini et al. recently described this technique with a particular emphasis on transvaginal approach, reporting that all 62 women included in the study obtained an adequate sample for histological analysis with no major complications registered.
They concluded that transvaginal US-guided biopsy is an adequate and cost-effective minimally invasive procedure through which potentially avoid unnecessary surgeries, and reduce long waiting times (44).
The role of US in the detection of recurrent disease after FSS for ESOC and borderline ovarian tumor (BOT)
According to international guidelines, thanks to its high sensitivity in the early detection of small volume ovarian lesions, a central role is awarded to TV-US in the follow up of patients affected by ESOC or BOT treated by FSS (4,5,11,45,46).
FSS is defined as the preservation of the uterus and at least a part of an ovary, associated to a complete staging procedure.
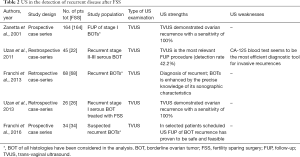
Recurrence rate of ESOC subjected to FSS is about 11.6% (ranging between 9.2% for FIGO stage IA to 14% for FIGO stage IC) (6). In the large series of patients reported by Bentivegna et al., 38% of patients recurred on the spared ovary, with a median time to recurrence of 43 months (range, 2–172 months) and a median follow-up of 186 months (range, 28–294 months) (6).
US is therefore essential for the early detection of secondary lesions developing on the spared ovary, in order to achieve the correct treatment.
Concerning BOT, recurrence rate has been described of up to 11% (47,48) with a malignant transformation rate of about 2–4% (2,49).
Considering that 10% of patients could relapse after more than 10–15 years (2,50-52), follow-up must be conducted for a longer period of time than for patients with OC (53).
Furthermore, scheduled timing of follow-up visits should be based on the presence of one or more specific negative prognostic factors such as advanced stage disease, the presence of invasive implants, residual tumor, micropapillary borderline and/or microinvasive tumor and incomplete surgical staging (54).
Follow-up strategies for BOT consist in a combination of clinical examination, TV/TA-US and dosage of CA-125 levels. Serum CA-125 levels and gynecological examination, in particular in recurrent stage I BOT, showed a very low detection rate, with 71% of patients with recurrent disease and CA-125 levels under the threshold of 35 U/mL (55).
For these reasons, TA and TV-US are currently considered the optimal techniques for the surveillance of patients with BOT treated with FSS, with a sensitivity of 100% (56,57).
This is possible because the majority of patients display local recurrences, as small size ovarian lesions (56), and have an extremely low risk of retroperitoneal recurrence, distant metastases or peritoneal spread (52).
Furthermore, the ability of US to early detect very small recurrences and to assess the precise amount of functioning ovarian parenchyma gives the possibility to offer a repeated FSS in a selected subset of childbearing-age patients (57,58).
BOT’s recurrence appears at US, within the speared ovary, as a unilocular-solid cyst (in case of serous tumors) with one or multiple papillary projections usually moderately to richly vascularized at CD, or as multilocular/multilocular-solid cysts (in case of mucinous borderline tumors). Interestingly, BOT’s recurrent lesions on the spared ovary mimic the US morphological features of the primary tumor, while pelvic recurrences of OC display completely different sonographic features from the primary tumor (55,56,59).
US is confirmed to be by far the best follow up tool for BOT, superior to CA-125 assay and certainly less hazardous, invasive, and expensive than CT scan. This leading role is not confirmed when the disease recurs with a diffuse peritoneal spread. As a matter of fact, when considering patients treated for an advanced-stage serous BOT, recurrence rate is about 27.5% and the recurrence pattern is more likely to be invasive (60).
In this specific subset of patients, CA-125 assay appeared to be the most appropriate tool for the identification of invasive recurrences, with a higher detection rate and the most serious impact on survival (60).
An additional sonographic parameter, that may be relevant in planning the best surgical management, is the growth rate trend of BOT recurrence.
Franchi et al. demonstrated that follow-up timing, cyst diameter and micropapillary patterns are significantly related to growth rate trend.
Moreover the growth rate of suspected recurrent lesion seems to vary according to size category at first US, ranging from a minimum of 0.06 mm/month for cysts <10 mm up to 1.92 mm/month for cysts >20 mm.
This could be useful in deciding which patient could benefit more from an intensive follow-up rather than immediate surgery. Optimal time for surgery seems to be when the recurrent tumor is large enough to be macroscopically detected, thus potentially reducing the risk of damaging the remaining healthy parenchyma in order to maximize the fertility/prognosis tradeoff (59).
The role of US as an intraoperative tool for localization of OC recurrence
Secondary cytoreduction for platinum sensitive OC patients, although extensively debated, have shown a significant survival advantage, especially in solitary and isolated lesions.
Preoperative imaging work up, including MRI, PET CT and CT scan, may help the surgeon to map the recurrent lesion and may lead to complete removal.
Due to its high feasibility and low cost, US can also be used as an intraoperative tool in order to find isolated secondary lesions previously mapped during preoperative imaging. The ability to provide a real-time high-resolution image of the region of interest, has recently established the role of intraoperative US (IO-US) in a variety of surgical procedures.
Not much data has been published on the role of IO-US in gynecological cancer.
As Mascilini et al. reported in 2018 (61), the use of intraoperative US in ROC helped the surgeon to correctly identify the previously visualized lesion and to perform and confirm a complete resection. In that series in fact, all patients were treated laparoscopically and the use of IO-US prevented from laparotomic conversion.
IO-US represent a promising tool also to achieve a complete laparotomic cytoreduction through the identification and subsequent targeted removal of suspicious cardiophrenic lymph nodes as described by Moro et al. (62).
In the field of FSS the use of IO-US has been recently investigated in terms of ovarian parenchyma sparing during surgery for recurrent disease, although only a few case reports have been published on this topic (63,64).
Further and prospective studies are needed to validate and explore all potential benefits of this innovative technique.
Conclusions
Based on this review of the literature, if performed by an expert sonographer, US seems to be an efficient tool in the early detection of ROC lesions, mainly when they occur in the pelvic region and during follow-up of fertility-sparing treated patients.
In these patients, the accuracy rate of US is high and adds useful information to the planning of optimized treatment, in determining whether should it be surgical or not.
The ability to precisely locate the lesion and the possible applications of the intraoperative US lead to a single-patient-fitted treatment, thus avoiding unnecessary surgery and related potential complications or leading the surgeon to a more precise excisional management.
The feasibility of the technique in outpatient setting, its non-invasiveness and its relatively low cost, suggest that US could be offered as a routine radiological examination during follow up of OC patients.
Lack of standardization methods and timing is still an open question.
Combination of non-invasive methods, such as US and CA-125, in addition to conventional staging exams, as CT scan or PET-CT, could lead to a faster detection of OC relapses during follow-up, and most of all to a tailored treatment strategy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Stefano Cianci) for the series “Ovarian Cancer Recurrence” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-357). The series “Ovarian Cancer Recurrence” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour Les Etudes Des Cancers De l'Ovaire (GINECO). Cancer 2009;115:1234-44. [Crossref] [PubMed]
- Gueli Alletti S, Capozzi VA, Rosati A, et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: a systematic review of the literature. Minerva Med 2019;110:341-57. [Crossref] [PubMed]
- Bentivegna E, Gouy S, Maulard A, et al. Fertility-sparing surgery in epithelial ovarian cancer: a systematic review of oncological issues. Ann Oncol 2016;27:1994-2004. [Crossref] [PubMed]
- Fruscio R, Corso S, Ceppi L, et al. Conservative management of earlystage epithelial ovarian cancer: results of a large retrospective series. Ann Oncol 2013;24:138-44. [Crossref] [PubMed]
- Bentivegna E, Fruscio R, Roussin S, et al. Long-term follow-up of patients with an isolated ovarian recurrence after conservative treatment of epithelial ovarian cancer: review of the results of an international multicenter study comprising 545 patients. Fertil Steril 2015;104:1319-24. [Crossref] [PubMed]
- Lheureux S, Braunstein M, Oza AM. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. Ca Cancer J Clin 2019;69:280-304. [PubMed]
- Trimbos JB, Parmar M, Vergote I, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst 2003;95:105-12. [Crossref] [PubMed]
- Laganà AS, Colonese F, Colonese E, et al. Cytogenetic Analysis of Epithelial Ovarian Cancer's Stem Cells: An Overview on New Diagnostic and Therapeutic Perspectives. Eur J Gynaecol Oncol 2015;36:495-505. [PubMed]
- Laganà AS, Sofo V, Vitale SG, et al. Epithelial ovarian cancer inherent resistance: May the pleiotropic interaction between reduced immunosurveillance and drug-resistant cells play a key role? Gynecol Oncol Rep 2016;18:57-8. [Crossref] [PubMed]
- NCCN Guidelines. Ovarian Cancer, Version 3 2019.
- Tanner EJ, Chi DS, Eisenhauer EL, et al. Surveillance for the detection of recurrent ovarian cancer: survival impact or lead-time bias? Gynecol Oncol 2010;117:336-40. [Crossref] [PubMed]
- Bhosale P, Peungjesada S, Wei W, et al. Clinical utility of positron emission tomography/computed tomography in the evaluation of suspected recurrent ovarian cancer in the setting of normal CA-125 levels. Int J Gynecol Cancer 2010;20:936-44. [Crossref] [PubMed]
- Du Bois A, Vergote I, Ferron G, et al. Randomized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ENGOT ov20. J Clin Oncol 2017;35:5501. [Crossref]
- Marchetti C, Rosati A, Scaletta G, et al. Secondary cytoreductive surgery in platinum-sensitive recurrent ovarian cancer before olaparib maintenance: Still getting any benefit? A case-control study. Gynecol Oncol 2019;155:400-5. [Crossref] [PubMed]
- Marchetti C, Rosati A, Scambia G, et al. Secondary cytoreduction in platinum-sensitive recurrent ovarian cancer: are we missing something? Ann Transl Med 2019;7:S372. [Crossref] [PubMed]
- Petrillo M, Zucchetti M, Cianci S, et al. Pharmacokinetics of cisplatin during open and minimally-invasive secondary cytoreductive surgery plus HIPEC in women with platinum-sensitive recurrent ovarian cancer: a prospective study. J Gynecol Oncol 2019;30:e59. [Crossref] [PubMed]
- Petrillo M, De Iaco P, Cianci S, et al. Long-Term Survival for Platinum-Sensitive Recurrent Ovarian Cancer Patients Treated with Secondary Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann Surg Oncol 2016;23:1660-5. [Crossref] [PubMed]
- Cianci S, Ronsini C, Vizzielli G, et al. Cytoreductive surgery followed by HIPEC repetition for secondary ovarian cancer recurrence. Updates Surg 2019;71:389-94. [Crossref] [PubMed]
- Cianci S, Abatini C, Fagotti A, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal malignancies using new hybrid CO2 system: preliminary experience in referral center. Updates Surg 2019;71:555-60. [Crossref] [PubMed]
- Vitale SG, Capriglione S, Zito G, et al. Management of endometrial, ovarian and cervical cancer in the elderly: current approach to a challenging condition. Arch Gynecol Obstet 2019;299:299-315. [Crossref] [PubMed]
- Schuurman MS, Kruitwagen RFPM, Portielje JEA, et al. Treatment and outcome of elderly patients with advanced stage ovarian cancer: A nationwide analysis. Gynecol Oncol 2018;149:270-4. [Crossref] [PubMed]
- Cianci S, Rumolo V, Rosati A, et al. Sarcopenia in Ovarian Cancer Patients, Oncologic Outcomes Revealing the Importance of Clinical Nutrition: Review of Literature. Curr Pharm Des 2019;25:2480-90. [Crossref] [PubMed]
- Gallotta V, Conte C, Giudice MT, et al. Secondary Laparoscopic Cytoreduction in Recurrent Ovarian Cancer: A Large, Single-Institution Experience. J Minim Invasive Gynecol 2018;25:644-50. [Crossref] [PubMed]
- Fagotti A, Costantini B, Gallotta V, et al. Minimally invasive secondary cytoreduction plus HIPEC versus open surgery plus HIPEC in isolated relapse from ovarian cancer: a retrospective cohort study on perioperative outcomes. J Minim Invasive Gynecol 2015;22:428-32. [Crossref] [PubMed]
- Cianci S, Tarascio M, Rosati A, et al. Sexual function and quality of life of patients affected by ovarian cancer. Minerva Med 2019;110:320-9. [Crossref] [PubMed]
- Gueli Alletti S, Vizzielli G, Lafuenti L, et al. Single-Institution Propensity-Matched Study to Evaluate the Psychological Effect of Minimally Invasive Interval Debulking Surgery Versus Standard Laparotomic Treatment: From Body to Mind and Back. J Minim Invasive Gynecol 2018;25:816-22. [Crossref] [PubMed]
- Králíčková M, Laganà AS, Ghezzi F, et al. Endometriosis and risk of ovarian cancer: what do we know? Arch Gynecol Obstet 2020;301:1-10. [Crossref] [PubMed]
- Tanase Y, Kawaguchi R, Takahama J, et al. Factors that Differentiate between Endometriosis-associated Ovarian Cancer and Benign Ovarian Endometriosis with Mural Nodules. Magn Reson Med Sci 2018;17:231-7. [Crossref] [PubMed]
- Timmerman D, Van Calster B, Testa A, et al. Predicting the risk of malignancy in adnexal masses based on the simple rules form the International Ovarian Tumor Analysis Group. Am J Obstet Gynecol 2016;214:424-37. [Crossref] [PubMed]
- Fischerova D, Cibula D. Ultrasound in gynecological cancer: is it time for re-evaluation of its uses? Curr Oncol Rep 2015;17:28. [Crossref] [PubMed]
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Testa AC, Ciampelli M, Mastromarino C, et al. Detection of central pelvic recurrent disease with transvaginal color Doppler ultrasound in women treated for gynecological malignancy. Ultrasound Obstet Gynecol 2002;19:490-5. [Crossref] [PubMed]
- Testa AC, Fruscella E, Ludovisi M, et al. The role of sonographic examination in the follow-up of gynecological neoplasms. Gynecol Oncol 2005;99:696-703. [Crossref] [PubMed]
- Shen ZY, Shen AJ, Yang SL, et al. Combination of Sonographic Morphology Score and Tumor Markers for Detecting Postoperative Recurrent Pelvic Ovarian Carcinoma: Compared With MRI Assessment. Ultrasound Q 2019;35:45-53. [Crossref] [PubMed]
- Meanwell CA, Rolfe EB, Blackledge G, et al. Recurrent female pelvic cancer: assessment with transrectal ultrasonography. Radiology 1987;162:278-81. [Crossref] [PubMed]
- Squillaci E, Salzani MC, Grandinetti ML, et al. Recurrence of ovarian and uterine neoplasms: diagnosis with transrectal US. Radiology 1988;169:355-8. [Crossref] [PubMed]
- Sugiyama T, Nishida T, Komai K, et al. Comparison of CA 125 assays with abdominopelvic computed tomography and transvaginal ultrasound in monitoring of ovarian cancer. Int J Gynaecol Obstet 1996;54:251-6. [Crossref] [PubMed]
- Fehm T, Heller F, Krämer S, et al. Evaluation of CA125, physical and radiological findings in follow-up of ovarian cancer patients. Anticancer Res 2005;25:1551-4. [PubMed]
- La Fianza A, Madonia L. Splenic metastases in gynaecologic cancer: clinical considerations, US, and CT diagnostic results. Radiol Med 2003;106:36-43. [PubMed]
- Fischerova D, Cibula D, Dundr P, et al. Ultrasound-guided tru-cut biopsy in the management of advanced abdomino-pelvic tumors. Int J Gynecol Cancer 2008;18:833-7. [Crossref] [PubMed]
- Zikan M, Fischerova D, Pinkavova I, et al. Ultrasound-guided tru-cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound Obstet Gynecol 2010;36:767-72. [Crossref] [PubMed]
- Mascilini F, Quagliozzi L, Moro F, et al. Role of transvaginal ultrasound-guided biopsy in gynecology. Int J Gynecol Cancer 2020;30:128-32. [Crossref] [PubMed]
- Satoh T, Hatae M, Watanabe Y, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol 2010;28:1727-32. [Crossref] [PubMed]
- Cadron I, Leunen K, Van Gorp T, et al. Management of borderline ovarian neoplasms. J Clin Oncol 2007;25:2928-37. [Crossref] [PubMed]
- Du Bois A, Ewald-Riegler N, du Bois O, et al. Borderline tumors of the ovary: A systematic review. Geburtsh Frauenheilk 2009;69:807-33. [Crossref]
- Creasman WT, Park R, Norris H, et al. Stage I borderline ovarian tumors. Obstet Gynecol 1982;59:93-6. [PubMed]
- Shih IeM, Kurman RJ. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am J Pathol 2004;164:1511-8. [Crossref] [PubMed]
- Seidman JD, Kurman RJ. Ovarian serous borderline tumors: A critical review of the literature with emphasis on prognostic indicators. Hum Pathol 2000;31:539-57. [Crossref] [PubMed]
- Silva EG, Gershenson DM, Malpica A, et al. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol 2006;30:1367-71. [Crossref] [PubMed]
- Rota SM, Zanetta G, Lissoni A, et al. The behavior of borderline ovarian tumors (BLT) with particular interest to persistence, recurrence and progression to invasive carcinoma: a prospective study on 339 cases. Int J Gynecol Cancer 1999;9:31.
- Benedet JL, Bender H, Jones H 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70:209-62. [Crossref] [PubMed]
- Fischerova D, Zikan M, Dundr P. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist 2012;17:1515-33. [Crossref] [PubMed]
- Franchi D, Boveri S, Fruscio R, et al. Imaging in gynecological disease (8): ultrasound characteristics of recurrent borderline ovarian tumors. Ultrasound Obstet Gynecol 2013;41:452-8. [Crossref] [PubMed]
- Zanetta G, Rota S, Lissoni A, et al. Ultrasound, physical examination, and CA 125 measurement for the detection of recurrence after conservative surgery for early borderline ovarian tumors. Gynecol Oncol 2001;81:63-6. [Crossref] [PubMed]
- Uzan C, Muller E, Kane A, et al. Fertility sparing treatment of recurrent stage I serous borderline ovarian tumours. Hum Reprod 2013;28:3222-6. [Crossref] [PubMed]
- Uzan C, Nikpayam M, Ribassin-Majed L, et al. Influence of histological subtypes on the risk of an invasive recurrence in a large series of stage I borderline ovarian tumor including 191 conservative treatments. Ann Oncol 2014;25:1312-9. [Crossref] [PubMed]
- Franchi D, Boveri S, Radice D, et al. Ultrasonographic diagnosis and longitudinal follow-up of recurrences after conservative surgery for borderline ovarian tumors. Am J Obstet Gynecol 2016;215:756.e1-756.e9. [Crossref] [PubMed]
- Uzan C, Kane A, Rey A, et al. How to follow up advanced-stage borderline tumours? Mode of diagnosis of recurrence in a large series stage II-III serous borderline tumours of the ovary. Ann Oncol 2011;22:631-5. [Crossref] [PubMed]
- Mascilini F, Quagliozzi L, Moro F, et al. Role of Intraoperative Ultrasound to Extend the Application of Minimally Invasive Surgery for Treatment of Recurrent Gynecologic Cancer. J Minim Invasive Gynecol 2018;25:848-54. [Crossref] [PubMed]
- Moro F, Uccella S, Testa AC, et al. Intraoperative Ultrasound-Guided Excision of Cardiophrenic Lymph Nodes in an Advanced Ovarian Cancer Patient. Int J Gynecol Cancer 2018;28:1672-5. [Crossref] [PubMed]
- Jones BP, Saso S, Farren J, et al. Intraoperative ultrasound-guided laparoscopic ovarian-tissue-preserving surgery for recurrent borderline ovarian tumor. Ultrasound Obstet Gynecol 2017;50:405-6. [Crossref] [PubMed]
- Mascilini F, Quagliozzi L, Bolomini G, et al. Intraoperative ultrasound through laparoscopic probe in fertility-sparing surgery for borderline ovarian tumor recurrence. Ultrasound Obstet Gynecol 2019;54:280-2. [Crossref] [PubMed]