Parathyroid carcinoma: a silent presentation
Introduction
Parathyroid carcinoma is the rarest endocrine malignancy and represents 1% of all cases of primary hyperparathyroidism (1). Patients often present with severe fatigue, nephrolithiasis, pathologic fractures, brown tumors, hypercalcemic crises, and if not recognized, obdurate hypercalcemia (2). Serum calcium and PTH levels are also much higher in parathyroid cancer patients than in patients with functional parathyroid adenoma; however, patients have been identified with non-functional non-secreting cancers, and they are often associated with a poor prognostic outcome. Non-functional parathyroid carcinomas represents 1.9% of all parathyroid carcinomas, whereas parathyroid adenomas are often benign and have no significant clinical presentation (3,4). Additionally, a palpable nodule located in the parathyroid region(s) is highly indicative of malignancy (2). The patient was completely asymptomatic with elevated free urine calcium, elevated parathyroid hormone (PTH) levels, and elevated serum calcium. She presented for surgical excision of a proposed hypersecreting adenoma. Reported cases of patients with recurrent laryngeal nerve palsy in patients without a past surgical history involving the neck is indicative of a more invasive lesion (1).
Case report
A 62-year-old Caucasian female presented to ambulatory surgery for a parathryoidectomy for hyperparathyroidism. The patient denied any neck masses or symptoms of throat tightness or dysphagia, and denied fatigue. No palpable neck mass was evident on physical examination. Her past medical history was significant for hypertension and Hepatitis C. Serum parathyroid hormone (PTH) level prior to surgery was 384.1 pg/mL and post-operative serum calcium level was 13.3 mg/dL. No other significant findings were evident on complete blood count, basic metabolic panel, or liver function testing.
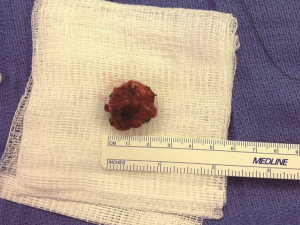
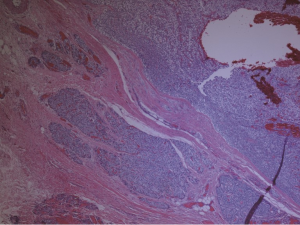
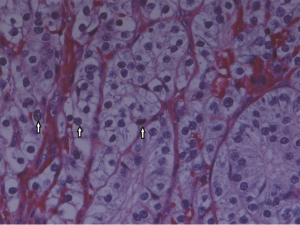
A preoperative ultrasound revealed a 2 cm mass in the right lower pole of the parathyroid region. A follow-up sestamibi displayed increased radionucleotide uptake in same right location with the impression of a parathyroid adenoma. Upon surgical exploration, the parathyroid mass was found to be grossly fibrotic and irregular in comparison to adjacent tissue (Figure 1). This tissue was removed with adjacent thyroid tissue, along with two adjacent lymph nodes. Histopathological examination revealed parathyroid carcinoma with contained capsular invasion with thyroid and lymph node specimens negative for malignancy (Figure 2). Large pleomorphic cells with scattered mitoses and vascular invasion was also evident (Figure 3).
Discussion
The diagnosis of parathyroid carcinoma typically relies on the patient’s clinical presentation, laboratory studies, imaging, and ultimately histopathology. In our case, the patient was absent of symptoms with only elevated serum calcium, elevated PTH level, elevated urinary calcium, and non-malignant imaging. Histopathological examination ultimately proved to be the final diagnosis. Ultrasound and Sestamibi scans are of additional benefit in localizing lesions and determining active vs. non-active lesions. The majority of parathyroid neoplasms are found in the inferior gland position, which is likely related to the different embryologic descent paths taken by the superior and inferior glands (5). Patients suspected of having parathyroid carcinoma should not undergo pre-operative biopsy procedures since the breaking away of cells in transit may serves as a nidus for ectopic dissemination of active parathyroid tissue (6,7). Measurement of intraoperative PTH level has been widely adopted to confirm removal of the hyperactive gland, and is considered satisfactory when the value is <50% of the pre-excision PTH level. However, the McGill University Thyroid Cancer Center has questioned this practice for adenomas localized preoperatively with two concordant imaging modalities, and instead recommends intraoperative PTH testing only if concordance between sestamibi scan, ultrasound and MRI cannot be established (8). In addition, intra-operative frozen section, although used frequently, has been shown to waste important tissue, and is therefore not recommended especially since tissue specimens are small and should be reserved for permanent section. However, in our case frozen sections allowed us to remove not just the mass but also adjacent tissue and lymph nodes to assess for local metastasis. Because of the difficulties in differentiating benign from malignant lesions, a set of standards set by Shantz and Castleman in 1973 has been adapted in ascertaining the diagnosis of a malignant parathyroid neoplasm. These include: mitoses within tumor parenchymal cells, vascular or capsular invasion by tumor cells, and a homogenous group of chief cells in a lobular pattern separated by thick fibrous septae (9,10). The parathyroid mass in our patient was grossly adhesed to the underlying thyroid tissue indicating heavy capsular invasion, the usual hallmark of parathyroid malignancy. In a large case series of 358 people, it was found that the most common sites of invasion, in descending order, were: ipsilateral thyroid gland, infrahyoid muscles, ipsilateral recurrent laryngeal nerve, esophagus, and trachea (3).
Molecular pathogenesis of parathyroid carcinoma has in part been revealed through studying Hyperparathyroidism-Jaw Tumor (HP-JT) syndrome. HP-JT is a rare autosomal dominant disease in which patients develop ossifying bone tumors of the maxillary and/or mandibular regions in conjunction with primary hyperparathyroidism, renal masses, and uterine masses (11). About 15% of the parathyroid lesions causing hyperparathyroidism in HP-JT syndrome are parathyroid carcinomas (12). A study of 14 families with HP-JT syndrome revealed germline inactivating mutations in the HRPT2/CDC73 tumor suppressor gene (13). The gene codes for a protein parafibromin which has been implicated in a variety of regulatory processes as well as histone methylation of promoter regions of various genes (14). Multiple studies have reported HRPT2/CDC73 gene mutations even in sporadic parathyroid carcinomas, validating the role of this mutation in development of parathyroid malignancy (15,16). Supporting the malignant nature of this mutation, other studies examined benign parathyroid lesions including adenomas, and/or hyperplasias for evidence of the mutation, with no identifiable genetic aberrations (15,17). Although meta-analyses have indicated an incidence of less than 1% of the mutation in benign parathyroid lesions (17). This data supports the use of genetic testing in parathyroid lesions suspected for malignancy. Due to different histopathological approaches used to diagnose parathyroid carcinoma, identifying a HPRT2/CDC73 mutation would be a definitive clue to a malignant parathyroid lesion. Case reports of histologically benign but metastatic parathyroid lesions have been reported that tested positive for the mutation further advocating the use of genetic testing in suspected parathyroid malignancy (18). In addition, it is recommended to test all parathyroid carcinoma confirmed patients for the mutation because up to one in five patients will have hidden HP-JT syndrome (16).
Multiple endocrine neoplasia (MEN)-1 syndrome is a rare autosomal dominant disease characterized primarily by a constellation of parathyroid adenomas, pituitary adenomas, and pancreatic tumors. The incidence of parathyroid carcinoma in MEN-1 patients is extremely rare; however, novel germline mutations in the MEN-1 gene have been isolated which may validate genetic testing in select individuals presenting with parathyroid carcinoma for the MEN-1 syndrome (19).
Treatment of parathyroid carcinoma is dependent on the stage of diagnosis. An en-bloc resection of the ipsilateral thyroid tissue with associated parathyroid lesions is recommended to decrease rate of recurrence. In the same case series of 358 en bloc resections, results showed an 8% decrease in local recurrence as compared to a 51% rate of local recurrence in patients who received just removal of the involved parathyroid gland(s) (3). Although this is recommended, our diagnosis was hinted by intra-operative examination and confirmed by permanent section where no evidence of further invasion was evident; therefore, a conservative parathyroidectomy was performed. Cervical lymph node metastasis is rare with presence in less than one in five affected patients; however, it is advised that the tracheoesophageal groove with level VI nodes be explored and removed with further dissection only if gross metastatic changes to other chains of lymph nodes is present (1,20,21). When parathyroid carcinoma is metastatic or unresectable, the resulting hypercalcemia is typically treated with bisphosphonates and calcium receptor agonists; however, moderate success using dacarbazine and denosumab has been reported for those patients with a refractory response (22).
Acknowledgements
Disclosure: This case was submitted as a case report poster for the American College of Osteopathic Surgeons annual meeting September 2014. No other financial or academic disclosures exist.
References
- Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab 2001;86:485-93. [PubMed]
- Dilli A, Gultekin SS, Ayaz UY, et al. Parathyroid carcinoma. JBR-BTR 2013;96:224-5. [PubMed]
- Koea JB, Shaw JH. Parathyroid cancer: biology and management. Surg Oncol 1999;8:155-65. [PubMed]
- Sharretts JM, Kebebew E, Simonds WF. Parathyroid cancer. Semin Oncol 2010;37:580-90. [PubMed]
- Goldner B, Lee B, Stabile BE. The unequal distribution of parathyroid neoplasms in male patients. Am Surg 2013;79:1022-5. [PubMed]
- Spinelli C, Bonadio AG, Berti P, et al. Cutaneous spreading of parathyroid carcinoma after fine needle aspiration cytology. J Endocrinol Invest 2000;23:255-7. [PubMed]
- Kebebew E, Arici C, Duh QY, et al. Localization and reoperation results for persistent and recurrent parathyroid carcinoma. Arch Surg 2001;136:878-85. [PubMed]
- Zawawi F, Mlynarek AM, Cantor A, et al. Intraoperative parathyroid hormone level in parathyroidectomy: which patients benefit from it? J Otolaryngol Head Neck Surg 2013;42:56. [PubMed]
- Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer 1973;31:600-5. [PubMed]
- Marcus S, Holley AC, Persad P, et al. Hypercalcemia in an elderly patient. JAMA Otolaryngol Head Neck Surg 2014;140:169-70. [PubMed]
- Kutcher MR, Rigby MH, Bullock M, et al. Hyperparathyroidism-jaw tumor syndrome. Head Neck 2013;35:E175-7. [PubMed]
- Carlson D. Parathyroid pathology: hyperparathyroidism and parathyroid tumors. Arch Pathol Lab Med 2010;134:1639-44. [PubMed]
- Carpten JD, Robbins CM, Villablanca A, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet 2002;32:676-80. [PubMed]
- Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, et al. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol Cell Biol 2005;25:612-20. [PubMed]
- Howell VM, Haven CJ, Kahnoski K, et al. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet 2003;40:657-63. [PubMed]
- Shattuck TM, Välimäki S, Obara T, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med 2003;349:1722-9. [PubMed]
- Krebs LJ, Shattuck TM, Arnold A. HRPT2 mutational analysis of typical sporadic parathyroid adenomas. J Clin Endocrinol Metab 2005;90:5015-7. [PubMed]
- Sarquis MS, Silveira LG, Pimenta FJ, et al. Familial hyperparathyroidism: surgical outcome after 30 years of follow-up in three families with germline HRPT2 mutations. Surgery 2008;143:630-40. [PubMed]
- Juodelė L, Serapinas D, Sabaliauskas G, et al. Carcinoma of two parathyroid glands caused by a novel MEN1 gene mutation - a rare feature of the MEN 1 syndrome. Medicina (Kaunas) 2011;47:635-9. [PubMed]
- Ricci G, Assenza M, Barreca M, et al. Parathyroid carcinoma: the importance of high clinical suspicion for a correct management. Int J Surg Oncol 2012;2012:649148.
- Pelizzo MR, Piotto A, Bergamasco A, et al. Parathyroid carcinoma. Therapeutic strategies derived from 20 years of experience. Minerva Endocrinol 2001;26:23-9. [PubMed]
- Vellanki P, Lange K, Elaraj D, et al. Denosumab for management of parathyroid carcinoma-mediated hypercalcemia. J Clin Endocrinol Metab 2014;99:387-90. [PubMed]


