Dietery factor obesity microenvironnement and breast cancer
Introduction
Epidemiological, clinical and experimental studies have raised a considerable amount of factors leading to the occurrence of breast cancer whose diet which represents a significant proportion. However, a number of controversies and unknowns remain regarding the impact of dietary factors on the risk of breast cancer. Studies in the field “Food and Breast Cancer” in recent years have faced some difficulties because on one hand, it is difficult to accurately estimate dietary intakes of individuals, which is a major limitation of epidemiological studies, and the other breast cancer is a complex multifactorial illness caused by cellular processes that genotype depends not only on tumor cells but also on the interactions between these cells and their microenvironment during different stages of disease progression.
Adipocytes represent the predominant cell types in the microenvironment of some tumors such as breast cancer (1).
Many studies suggest that adipocytes could play an important role in the early stages of mammary carcinogenesis via their ability to secrete growth factors, cytokines or many adipokines, proteases and certain compounds of the extracellular matrix such as collagen VI (2).
In addition, clinical studies and epidemiological surveys show that obesity is a factor of poor prognosis in many cancers including breast cancer in postmenopausal women (3).
Moreover, the acquisition of basic knowledge on the mechanisms of oncogenesis related to tumor microenvironment is an essential scientific direction for the improvement of current therapies and the development of new therapeutic approaches.
The objective of our work is to analyze the relation between food consumption, obesity and breast cancer in a group of Moroccan women with breast cancer and compare it to controls. Lately, the advances in the role of the adipocyte in carcinogenesis will be discussed.
Patients and methods
Recruitment of cases and controls
We conducted a case-control study at The National Institute of Oncology Sidi Mohamed Ben Abdallah Rabat in which we included a population of Moroccan women (collected between December 2008 and December 2010) we have divided into two groups, a group of women with breast cancer, and a group of breast cancer-free controls.
Inclusion criteria
Recruitment of cases of breast cancer was based on a diagnosis of breast cancer confirmed by mammography, biopsy and/or surgery by specialists of the National Institute of Oncology. Women recruited and respondents must be of all ages and must be patients newly diagnosed as breast cancer. Controls recruited at the same Institute within the framework of the cancer screening campaign organized by government authorities after having undergone a mammography that showed no signs of breast cancer.
To ensure maximum comparability between cases and controls and to ensure representative of the study population each case was matched with one control of the same age we did correspond to each case a witness of the same age. The subjects recruited for this study were aged between 22 and 75 years.
These women were interviewed for epidemiological, tumor information and their eating habits. The epidemiological data collected included (I) age; (II) place of residence; (III) menopausal status and (IV) body mass.
Tumor withheld information was the date of disease, stage of disease and the terms of the discovery of the disease, this information relates only to the patient population.
Exemption criteria
We excluded from the study all women with chronic pathology associated, such as high blood pressure, diabetes and coronary heart disease, women who not within the age rank of 22-75 years and patients who were prescribed a treatment (chemotherapy, radiation therapy, hormone therapy).
Food questionnaire
This is a questionnaire on the frequency of consumption during a given and for a fixed period food series. The questionnaire is oriented foods commonly consumed and frequency. This type of survey does not provide a quantitative assessment of dietary intake or on actual consumption but allows for an assessment of the habitual consumption of food and has the advantages of being practicable on all samples and results no changes in the diet of respondents. During our investigation we sought to determine the frequency of consumption of each food (per month, per week) to a predetermined list with a food frequency questionnaire that was administered in a standardized manner for cases and controls. Participants were asked to indicate the frequency of consumption of each food item requested in the questionnaire by checking the usual frequency of consumption which can be: no consumption, 1 time per month, 2 times per month, 3 times per month, 1 time per week, 2 times per week, 3 times per week, 4 times per week, 5 times per week, 6 times per week, 7 times per week. Then we stratified the frequency of consumption frequency into four categories: (I) never in patients who do not consume the food requested; (II) less than once a week in patients who consume the food required (1 time per month, 2 times per month, 3 times per month); (III) less than or equal to 3 times a week for patients who consume food requested (1 time per week, 2 times per week, 3 times per week) finally (IV) greater than 3 times a week for patients who consume food requested (4 times per week, 5 times per week, weekly 6 times, 7 times per week). Our examination concerned the foods high in animal fats such as red meat, processed meat, poultry, eggs, fish, foods rich in fiber such as fruits, vegetables and cereals, calcium foods such as milk and milk products.
To meet the objective of our study we established four groups of women according to the body mass index (BMI) (I) low group; (II) normal group; (III) overweight group; (IV) group obesity and we seek to know the four groups in each population the frequency of consumption of certain foods implicated in several cancers including breast carcinogenesis and having made the subject of several epidemiological studies such as red meats and processed meat.
Statistical analysis
Epidemiological parameters collected on survey forms were entered and analyzed using Statistical Package for Social Sciences (SPSS) software Version 13.0 then evaluated initially by a statistical analysis using the Pearson chi2 test, the results are expressed as numbers and percentages. Study variables collected were tested vis-à-vis the possible association with breast cancer. Initially, each variable was assessed independently in a univariate analysis adjusted on age then when the next step all variables were fed into a multiple regression model to assess the odds ratios (OR). A threshold of P<0.05 was considered significant.
Ethical considerations
Respect for the anonymity and confidentiality of information was strictly adhered to. Informed consent was signed before the inclusion of women in the study.
Results
General settings of the population (Table 1)
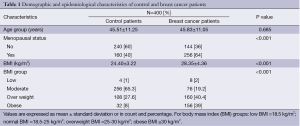
Full table
In this study we included eight hundred women who were divided into two populations, a population of 400 women with breast cancer and a population of 400 women who were breast cancer-free. Table 1 shows that there does not exist statistically significant difference in the average age of the two populations (45.51±11.25 years in the group of controls and 45.83±11.05 years in the group of patients P=0.685). BMI was significantly higher in the patient group (28.35±4.36 vs. 24.4±3.22 kg/m2, P<0.001). The percentage of postmenopausal women was statistically significantly higher in the patient group (64% vs. 40%, P<0.001).
Analysis of information tumor patient women shows that the mode of the lesion was discovered by palpation in 180 cases (45%) and after a mammogram in 220 cases (55%). Histological size lesion was higher than 1 cm in 150 cases (37.5%) and the degree of Scarff-Bloom-Richardson (SBR) was I or II in 240 cases (60%).
Result dietary survey
The nutritional data collection was performed using a food frequency questionnaire which concerned the control population and the patient population. During this investigation we were interested in knowing the frequency of consumption of foods made with the object of several studies. The interview included particularly questions related to foods rich in animal fat, rich in fiber and calcium foods. The analysis of Table 2 showing the distribution of patient and control women according to a frequency of consumption of foods rich in animal fat allowed us to make a descriptive analysis of the food trend of the population study and noted variations in the frequency of consumption of foods rich in lipid material such as:
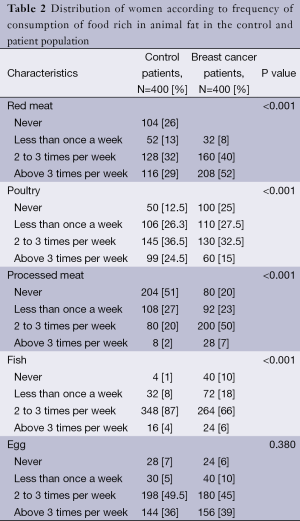
Full table
- Red meat: the frequency of consumption of meat was statistically higher in the patient group (52% of patients consumed meat 4 to 7 times per week versus 29% of control women and 40% of patients consumed meat 2 to 3 times per week versus 32% of control women, P<0.001).
- Processed meat: the frequency of consumption of processed meat was statistically higher in the patient group (7% of patients consumed sausage 4 to 7 times per week versus 2% of control women and 50% of patients consumed sausage 2 to 3 times per week versus 20% of control women, P<0.001).
- Eggs: the frequency of consumption of eggs was relatively similar in both groups of women (P=0.380).
Moreover, the consumption of poultry and fish was statistically reduced in the patient group: 15% of patients consumed poultry 4 to 7 times per week versus 24.5% of control women and 66% of patients consumed fish 2 to 3 times per week versus 87% of control women (P<0.001).
The analysis of Table 3 describing the distribution of patients and control group according to frequency of consumption of high-fiber foods like vegetables, fruits and cereals was also significantly decreased in the population with breast cancer than in the control population.
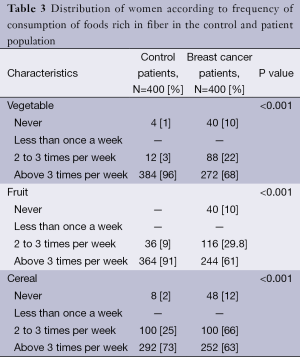
Full table
However the analysis of Table 4 showed that the frequency of drinks milk and dairy products was relatively similar between the two groups of women studied.
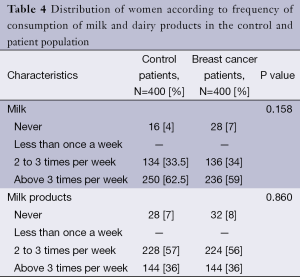
Full table
In addition to that, we were interested in comparing the frequency of consumption of certain implicated in mammary carcinogenesis as red meats and processed meat between the two populations of women according to BMI foods. The analysis of Figure 1 showed that the percentage of female patients who consumed red meat 4 to 7 times per week was statistically higher in the obese and the overweight group compared with control women of the same BMI group and having the same frequency consumption as follows:
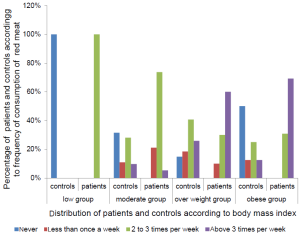
In the obese group the percentage of women who ate meat 4 to 7 times per week was significantly higher in the patient population (69.2% of female patients versus 12.5% of control women, P<0.001).
In the group of overweight the percentage of women who ate meat 4 to 7 times per week was statistically higher in the patient population (60% of female patients versus 25.9% control, P<0.001).
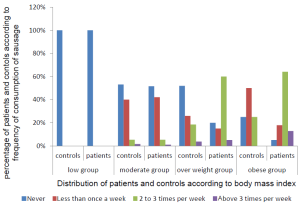
The Figure 2 showed that the frequency of consumption of meats in the low group and the moderate group was similar in both populations. However, in the overweight group the percentage of female patients who consumed meats 1 to 3 times per week was significantly higher (60% of female patients versus 18.5% of control women, P<0.001) and the percentage of female patients who consumed cured meats 4 to 7 times per week was significantly higher (12.8% versus 0% of control women).
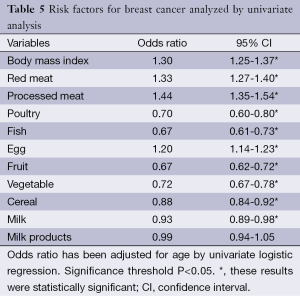
In univariate analysis (Table 5) we found out that BMI increased by 1.3 the risk of breast cancer [95% confidence interval (CI), 1.25-1.37] and this increase was statistically significant (P<0.001), red meat multiply by 1.33 the risk of breast cancer (95% CI, 1.27-1.40), processed meat multiply by 1.44 the risk of cancer (95% CI, 1.3-1.54), and then the eggs multiply by 1.20 the risk of cancer (95% CI, 1.14-1.23). However other foods such as poultry, fish, vegetables, fruits, cereals and milk were identified as factors that significantly reduce the risk of breast cancer, so they are protective factors. With regard to dairy products, they have not been identified as a predictive risk of breast cancer.
Full table
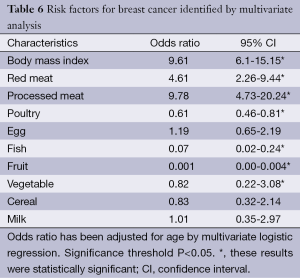
All significant variables found in the univariate analyses were included in the multivariate regression analysis (Table 6). The significantly elevated OR for breast cancer were associated with BMI (OR =9.61; 95% CI, 6.1-15.15), red meat (OR =4.61; 95% CI, 2.26-9.44) and processed meat (OR =9.78; 95% CI, 4.73-20.24). However consumption of fish (OR =0.07; 95% CI, 0.02-0.24), fruit (OR =0.001; 95% CI, 0.00-0.004), vegetable (OR =0.82; 95% CI, 0.22-3.08), and poultry (OR =0.61; 95% CI, 0.46-0.81), were a protective factor.
Full table
Discussion
In the present case-control study, we found several dietary factors associated with breast cancer in Moroccan women.
Firstly, the high foods of fat namely red meat and processed meat were significantly increased among the breast cancer women when compared with the control women (Table 2). In univariate analysis the high intake of red meats and processed meat increase the risk of breast cancer by 1.33 (95% CI, 1.27-1.40) and 1.44 (95% CI, 1.35-1.54), respectively.
Similarly, when multivariate analysis was performed, both red meat (OR =4.61; 95% CI, 2.26-9.44) and processed meat (OR =9.78; 95% CI, 4.73-20.24) were remain significantly increased in breast cancer, which implies that there factors may be positively associated with breast cancer in Moroccan woman. This may indicate that a high intake of fat increases the risk of breast cancer. Our results thus confirm reports of breast cancer association with red meat.
Other studies on various ethnic groups have not obtained consistent results on the effect of meat intake on breast-cancer risk. Contrary to our findings, a meta-analysis study on 351,041 women with breast cancer has not showed any association (4). However, a case control study on 250 Taiwanese women with breast cancer showed a high consumption of meat (5).
In addition, Taylor et al. showed a strong positive association of breast cancer with meat consumption in the UK menopausal women (6).
Recently, Cho et al. (7) evaluated the contribution of red meat and risk of breast cancer among premenopausal women (Nurses’ Health Study II). During 12 years of follow-up 90,659 premenopausal women, they noted a high risk only in women with positive estrogen receptors and progesterone. Several biological mechanisms may explain this positive association including: (I) a stimulation of estrogen receptors by heterocyclic amines (8); (II) action of exogenous hormones (used to stimulate the growth of livestock) (9); (III) action of heme iron content in red meat, improving tumor induction by estrogen (10,11); (IV) finally, it has been suggested that the fat content can increase the risk of breast cancer by increasing the circulating estrogen levels (12).
Another important finding of the present study is that, in the univariate analysis, the egg was positively associated with the presence of breast cancer in our population. Although this association did not persist in the multivariate analysis, possibly because of a lack of power, there was a clear effect of egg intake on breast-cancer risk.
In the other hand, a negative association between poultry, fish and breast cancer suggested a protective effect of their specific food items for breast cancer. Most of studies have found no significant association between consumption of poultry and this disease (13,14). In contrast a case-control study (114 cases and 280 controls) noted a decreased risk of breast cancer (15).
The protective effect of white meat can be explained by their amino acid content; they reinforce the action of the anti-tumor immunity. However, further studies are needed to confirm the protective effect of white meat. Studies conducted in vitro and in animals have shown that n-3 fatty acids of marine origin have inhibitory effects on the mammary tumor growth (16). Terry et al. have reported conflicting results (17). Recently, Engeset et al. (18) did not find any evidence of an association between fish intake and risk of breast cancer in European women. One limitation for the inconsistency may be lack of distinction between lean fish and fatty fish. Interestingly, we found a negative association between consumption of fruits and vegetables and breast cancer (Tables 5,6). This negative association is in agreement with data in the literature. Indeed many fruits and vegetables contain protective substances, such as fiber, antioxidants, minerals and other potential anti-cancer compounds, including isothiocyanates, indole-3-carbinol, flavonols, and ligands (19). Some vegetables seem neutralize enzymes that activate the triggering agents of cancer in the body and increase the other enzymes which reduce the presence of these elements carcinogenic activity.
Studies of associations between the consumption of fruits and vegetables and the incidence of breast cancer have concerned many case-controls and a limited number of cohort studies. Recent studies [2000-2004] have highlighted the antioxidant power of fruits and vegetables, and their properties to prevent the proliferation of cancer cells. The World Cancer Research Fund and the American Institute for Cancer Research believe that a diet rich in vegetables and fruits (more than 400 grams per day) can prevent at least 20% of all cancers (20).
However, a meta-analysis of studies on breast cancer risk and diet did not find a protective effect of the consumption of fruit and vegetables (21).
Some studies suggest that increased intake of dietary fiber may have a small protective effect against breast cancer, possibly by reducing blood levels of estrogen by binding effect in the intestinal lumen.
In our population, obesity was also found to be strongly linked to the presence of breast cancer. This association of obesity in women with breast cancer is now largely supported by several studies in the literature (22). In a prospective study involving 99,039 postmenopausal women, Ahn et al. (3) focused on the association between adiposity and weight change with incidence of breast cancer. The results of this study are (I) weight gain is partly responsible for breast cancer; (II) BMI was associated with an increased risk of breast cancer, especially among women who were not on menopausal hormone therapy, and (III) all women not on hormone replacement therapy and who gained weight between 35 and 50 years of age had a 1.4-fold increased risk of developing breast cancer (23). Breast cancer is a multifactorial disorder related with genetic, environmental and hormonal factors. Since fat intake, obesity and adipocyte are highly correlated, it has been proposed that cell types in the tumor-microenvironment contribute to the pathogenesis of breast cancer. The adipocyte is one of the most prominent cell types in the tumor-microenvironment of breast cancer. Recently, it has been demonstrated that adipocytes were considered as an endocrine cells producing adipocytokines including: vascular endothelial growth factor, hepatocyte growth factor, leptin, tumor necrosis factor-alpha, heparin-binding epidermal growth factor-like growth factor, and interleukin-6 (IL-6) (24). It is well known that the most of these cytokines have been associated with obesity and promote angiogenesis.
Therefore, mature adipocytes represent excellent candidates to influence tumor behavior through heterotypic signaling processes.
Several studies show that invading tumor cells are able to modify adipocytes phenotype, which, in turn, stimulate cancer cells aggressive behavior (25,26). In addition, peritumoral adipocytes exhibited a modified phenotype secrete sizeable amounts of proteases, including matrix metalloproteinase-11, and proinflammatory cytokines (IL-6, IL-1b). Dirat et al. show that proinflammatory cytokines, particularly IL-6, plays a key role in mediating adipocyte dependent invasive activity of tumor cells (26).
This study is rather in favor of an association between high fat intake, obesity and breast cancer, but it is difficult to interpret because on one hand we cannot rule out that unmeasured factor explains the observed relationship (factor of confusion). On the other hand in case-control studies, dietary habits are established retrospectively at the onset of the disease, so it can happen that patients report their consumption differently from controls (through differential lock). However, these results provide information that diet can increase the risk of breast cancer and may be used for further analysis of breast cancer susceptibility studies.
Acknowledgements
We thank all participants for taking part in this study.
Disclosure: The authors declare no conflict of interest.
References
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science 2002;296:1046-9. [PubMed]
- Rajala MW, Obici S, Scherer PE, et al. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest 2003;111:225-30. [PubMed]
- Ahn J, Schatzkin A, Lacey JV Jr, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med 2007;167:2091-102. [PubMed]
- Missmer SA, Smith-Warner SA, Spiegelman D, et al. Meat and dairy food consumption and breast cancer: a pooled analysis of cohort studies. Int J Epidemiol 2002;31:78-85. [PubMed]
- Stephany RW. Hormones in meat: different approaches in the EU and in the USA. APMIS Suppl 2001;(103): S357-63: discussion S363-4.
- Taylor EF, Burley VJ, Greenwood DC, et al. Meat consumption and risk of breast cancer in the UK Women’s Cohort Study. Br J Cancer 2007;96:1139-46. [PubMed]
- Cho E, Chen WY, Hunter DJ, et al. Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med 2006;166:2253-59. [PubMed]
- Snyderwine EG. Mammary gland carcinogenesis by 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine in rats: possible mechanisms. Cancer Lett 1999;143:211-5. [PubMed]
- Carpenter CE, Mahoney AW. Contributions of heme and nonheme iron to human nutrition. Crit Rev Food Sci Nutr 1992;31:333-67. [PubMed]
- Wyllie S, Liehr JG. Enhancement of estrogen-induced renal tumorigenesis in hamsters by dietary iron. Carcinogenesis 1998;19:1285-90. [PubMed]
- Liehr JG, Jones JS. Role of iron in estrogen-induced cancer. Curr Med Chem 2001;8:839-49. [PubMed]
- Cho E, Spiegelman D, Hunter DJ, et al. Premenopausal fat intake and risk of breast cancer. J Natl Cancer Inst 2003;95:1079-85. [PubMed]
- Toniolo P, Riboli E, Shore RE, et al. Consumption of meat, animal products, protein, and fat and risk of breast cancer: a prospective cohort study in New York. Epidemiology 1994;5:391-7. [PubMed]
- Franceschi S, Favero A, La Vecchia C, et al. Influence of food groups and food diversity on breast cancer risk in Italy. Int J Cancer 1995;63:785-9. [PubMed]
- Delfino RJ, Sinha R, Smith C, et al. Breast cancer, heterocyclic aromatic amines from meat and N-acetyltransferase 2 genotype. Carcinogenesis 2000;21:607-15. [PubMed]
- Rose DP, Connolly JM. Regulation of tumor angiogenesis by dietary fatty acids and eicosanoids. Nutr Cancer 2000;37:119-27. [PubMed]
- Terry PD, Rohan TE, Wolk A. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr 2003;77:532-43. [PubMed]
- Engeset D, Alsaker E, Lund E, et al. Fish consumption and breast cancer risk. The European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 2006;119:175-82. [PubMed]
- Terry P, Suzuki R, Hu FB, et al. A prospective study of major dietary patterns and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2001;10:1281-5. [PubMed]
- World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition and the prevention of cancer: a global perspective. American Institute for Cancer Research, Washington, DC 1997.
- Gandini S, Merzenich H, Robortson C, et al. Meta-analysis of studies on breast cancer risk and diet: the role of fruit and vegetable consumption and the intake of associated micronutrients. Eur J Cancer 2000;36:636-46. [PubMed]
- Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 2004;111:762-71. [PubMed]
- Laamiri FZ, Otmani A, Ahid S, et al. Lipid profile among Moroccan overweight women and breast cancer: a case-control study. Int J Gen Med 2013;6:439-45. [PubMed]
- Rajala MW, Scherer PE. Minireview: The adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 2003;144:3765-73. [PubMed]
- Motrescu ER, Rio MC. Cancer cells, adipocytes and matrix metalloproteinase 11: a vicious tumor progression cycle. Biol Chem 2008;389:1037-41. [PubMed]
- Dirat B, Bochet L, Dabek M, et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res 2011;71:2455-65. [PubMed]


