The impact of radiation on lymphedema: a review of the literature
Introduction
Radiation therapy (RT) has a long-established clinical history as a treatment modality in oncology and can extend disease-free survival, overall survival and reduce regional recurrence (1,2). However, RT carries a risk of nerve injury, impaired mobility, fibrosis, chronic pain, lymphedema, telangiectasia, and bone fractures (2). When applied in the thoracic cavity, RT can precipitate pulmonary fibrosis and ischemic heart disease as well (2). One of the most common morbidities of RT is lymphedema (1). In breast cancer, lymphedema affects 1 in 4 patients who undergo RT (3).
Lymphedema is a result of localized fluid retention and tissue swelling secondary to a dysfunction in the lymphatic drainage (3). It is defined by the accumulation of protein rich fluid, resulting in chronic inflammation and reactive fibrosis (4). The lymphatic system maintains fluid equilibrium in interstitial tissue as it continuously drains to the blood stream (3). Lymphedema has immunologic, psychologic, and functional side effects and increases the risk for infection and some malignancies (4,5). This review will examine the role of RT in upper and lower extremity lymphedema.
Methods
An online literature search was explored to assess the role of RT in the progression towards lymphedema with a focus on pelvic and breast malignancies. PubMed and MEDLINE were utilized to identify articles in English that investigated or commented on RT and lymphedema up until November 2019. Keywords queried included a various arrangement of the following terms: “radiation”, “radiation therapy”, “lower extremity lymphedema”, “gynecological malignancy”, “breast malignancy”, “reactive fibrosis”, “upper extremity lymphedema”, “breast cancer radiation therapy”, “ovarian cancer radiation therapy” and “endometrial cancer radiation therapy”. Eligibility criteria excluded articles that reported lymphedema progression without the use of RT, abstracts, case reports, presentations, as well as papers not written in English. Additional queries were performed based on relevant references of the searched articles as well.
Etiology and pathophysiology of radiation associated lymphedema
The lymphatic system consists of an intricate open network of lymph nodes, ducts and sinuses whose primary function is to drain excess interstitial fluid and other particulate matter such as colloid protein that are too large to enter directly into the blood stream (6). The lymphatic system also has an important role immunologically, as lymph nodes are important foci for T and B cells (6,7). The lymphatic system is structured such that afferent lymphatic vessels supply the lymph node with lymph, and lymphocytes are exposed to the lymphatic fluid as it flows through out the node which can activate the adaptive immune system when an antigen is detected (6-8).
There are two main types of lymphatic vessels: pre-collecting and collecting (9). The pre-collecting lymphatics are the smallest initial lymphatic vessels (9). The functions of the pre-collecting lymphatic vessels are to absorb and propel fluid, as they have a discontinuous basal lamina allowing for fluid absorption as well as a spontaneously contracting smooth muscle layer propelling lymph (10). The main afferent lymphatic vessel to the lymph node is the collecting vessel; the collecting lymphatic vessel contains smooth muscle and valves that contract further providing propulsion of lymph fluid, similar to a pump (11). Normally, lymph follows a one-way system designed to return contents into the blood stream with lymphatic nodes that filter this fluid (12). Nodal basins are concentrated in the groin, axilla, and neck (6,7,9). If flow of lymph is disrupted then the network passageways are disrupted and congestion can occur (12). Surgery and radiation heighten risk by directly injuring lymphatic vessels with subsequent reduction to the carrying capacity of the lymphatic vessels. This mechanical insufficiency results in leakage of protein rich fluid causing lymphedema (6,12).
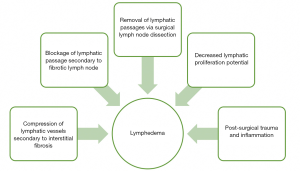
The immediate effect of direct radiation energy on lymphatic vessels is minimal as demonstrated in both in vitro and in vivo studies, as structural and functional integrity are maintained (13,14). Damage to the lymphatic vessels occurs in a delayed fashion after radiation as the surrounding tissue develops into dense fibrous tissue that compresses and blocks lymphatic flow (12,13). In addition, lymphatic proliferation is inhibited by RT preventing compensatory lymphatic vessel growth and further promoting lymphedema formation (13). Unlike lymphatic vessels which are insensitive to radiation, lymph nodes are highly radiosensitive (13). In response to radiation, lymph nodes will first become depleted of lymphocytes then undergo fatty change and ultimately fibrosis. Fibrosis of lymph nodes significantly alters their ability to filter lymphatic fluid, as well as increases pressure proximally which promotes lymphedema (15). Furthermore, there is a stronger propensity for the lymph node to transform into fibrous tissue after RT if the nodal basin had contained regional metastasis. Therefore, patients with lymph node metastasis undergoing RT are at an increased risk for lymphedema compared to irradiated patients without lymph node involvement (14,15). In summary, patients with radiation will have lymph node fibrosis with mechanical insufficiency of vessels as well as decreased proliferative capability. These histologic changes coupled with post-surgical inflammation and nodal resection can significantly promote the production of lymphedema as seen in Figure 1. This persistent swelling of lymphatic fluid can lead to fibrosis and increased inflammation as it is an ideal medium for cellulitis and lymphangitis (12).
Role of radiation in iatrogenic lymphedema
Upper limb lymphedema and breast RT
RT increase the risk of lymphedema for breast cancer (16-19). Johansen et al. found that post mastectomy RT increases the risk of lymphedema by nearly 5 times (20,21). Furthermore, when RT is coupled with lymph node dissection the risk increases to nearly 10 times; this effect increases with more extensive the lymph node dissection (5,22-24). Axillary lymph node sampling with RT to a partial or total lymphadenectomy with RT increases the risk of lymphedema from 9% to over 40% (16,17,23,25,26). Axillary lymph node dissection with radiation significantly increases the likelihood of developing lymphedema (20,27).
The specific radiation technique impacts on lymphedema risk as well. Patients exposed to tangential photon radiation, where the breast is wedged between two opposing tangential radiation fields, have a higher risk of lymphedema compared to patients undergoing diffuse chest wall electron beams, in which there is direct application of high energy electrons to the breast (22,28). Furthermore, there is a positive association of lymphedema with increasing total dose of radiation, overlapping radiation fields, and posterior axillary boost radiation (29).
Specific anatomic regions are at increased risk for developing lymphedema than others. Radiation of upper axillary nodal basins at level I, located between the latissimus dorsi to the lateral aspect of the pectoralis minor, and level II, posterior to the pectoralis minor, increased the risk for lymphedema as they contain a higher concentration of lymph nodes (30). More advanced techniques that limit overlapping radiation fields may lessen the risk associated with RT (22).
Volume and distribution of axillary tissue irradiated are independent risk factors for developing lymphedema. Distributing the radiation field such that it excludes axillary levels I/II even if it extends beyond one third of the humeral head reduces the incidence of lymphedema from 37% to 7% in a 5 year period, without increasing the risk of disease reoccurrence (30). Likewise, irradiation of large volumes of axillary tissue correlated with increased lymphedema as more draining passageways are destroyed secondary to the effects of RT (20). In addition to RT technique, systemic risk factors such as obesity increase risk of lymphedema. A body mass index >30 increases the risk of lymphedema development in post mastectomy RT by almost 3 times compared to a non-obese patient (31).
Lower limb lymphedema and gynecological RT
RT has also been found to increase the risk of lower limb lymphedema in patients with gynecological cancers such as ovarian, vulvar, and endometrial carcinoma (32-35). Lower limb lymphedema follows dysfunction in the pelvic and inguinal nodes (8). Post-operative RT in this region increases lymphedema risk by up to 40% (34,36-39). Unlike axillary RT, one study found that neither radiation dose nor length of delivery affect lymphedema rates (33). However, RT delivery technique does seem to impact lower limb lymphedema rates, as external beam RT was associated with increased risk of lymphedema compared to vaginal brachytherapy, where the radiation is delivered from inside the vagina.
For most gynecological cancers there are two modes of RT: external beam radiation and brachytherapy. Vaginal brachytherapy is able to provide targeted high dose radiation to gynecological malignancies while shielding nearby organs from unnecessary radiation given that radiation doses decreases by the inverse of the distance from the source squared (40). External beam radiation is provided as intensity modulated RT (IMRT) utilizing multiple external targeted low dosage radiation beams to concentrate at the tumor foci sparing normal tissue (41). However, IMRT involves larger radiation fields and despite it theoretically sparing normal tissue has been associated with “radiation induced second cancers” (42). Depending on the type, stage and location of the tumor these two RT modalities are often used in conjunction with chemotherapy (43). The risk associated with lower limb lymphedema in patients receiving vaginal brachytherapy has been estimated at 11% compared to a risk of 71% following pelvic external beam RT (44-47). Delivering RT through pelvic external beam increases risk of lower extremity lymphedema by five times (34).
Benefits of RT
Overall, the benefits of RT greatly outweigh the risks for appropriately selected patients. Ragaz et al. discovered a 33% reduction in recurrence and a 29% reduction in mortality among women with stage I/II breast cancer who received RT with chemotherapy compared to those who received chemotherapy alone after mastectomy (17). Several cancer institutes in North America such as in the United States and Canada supports RT in early stage breast cancer with high risk node metastasis because of this documented reduction in cancer recurrence; and in the United States, RT is an integral component in breast conserving therapy due to its increased benefits when compared to just chemotherapy alone (48-50). Yao et al. studied over 20,000 patients with triple negative breast cancer, and found that patients with RT had a smaller tumor size, better breast cancer specific survival and overall survival rates when compared to patients with no RT (51). Likewise, gynecological RT also has a significant positive impact on increased survival and reduced local recurrence (52-54).
Clinical diagnosis of radiation induced lymphedema
Given the lack of precise consensus on definitions or grading systems for lymphedema, clinicians and investigators have used a wide variety of independently developed, subjective, and objective diagnostic criteria. Lack of consensus has created inconsistencies in the literature on the definition of lymphedema as well as diagnosis techniques (55-57). For instance, the widely used arm circumference technique to assess upper extremity lymphedema has been shown to be highly inaccurate (23). While taking an accurate history and conducting a thorough physical examination are critical in diagnosing lymphedema, more objective measures need to be utilized. There are several recently published methods utilized to diagnose lymphedema.
Objective techniques used to diagnose lymphedema include ultrasound, tonometry, bioimpedance, and optoelectric perometry. Tonometry measures tissue pressure by evaluating the resistance of the tissues when compressed. The degree of compressibility has been correlated with the degree of lymphedema. Since there are no standard protocols, this technique has low reliability (58,59). Bio-Impedance is a non-invasive technique that directly measures lymph fluid volume through the impedance to an electrical current (55,58,60-62). Perometer is a noninvasive optoelectronic sensor that uses infrared light to measure the lymph volume of the limb (63-66). It is highly sensitive and reducible and can detect subclinical lymphedema within 2–3 minutes (63,65-68).
Radiologic studies include lymphoscintigraphy (LSG), magnetic resonance imaging (MRI), MR lymphangiography, fluorescein lymphangiography, near-infrared fluorescence imaging and computed tomography (CT). These imaging techniques can also be used to further support the diagnosis in complex presentations as is common for irradiated cancer patients. These imaging studies assess the lymphatic system either directly or indirectly by assessing function. However, most diagnose lymphedema by assessing the unique sequalae associated with lymphatic dysfunction, such as the development of fibrosis or impedance (68). Many consider the most objective standard method to diagnose lymphedema is through LSG.
Common to review articles, there are several limitations to this paper. Only journals published in the English language were assessed in this review. In addition, there is a possible bias in interpreting the results discussed in each article. While this review article discussed various radiation delivering modalities, specific RT protocols and their effect on lymphedema progression were not assessed in detail. Further studies should focus on the impact of differing RT protocols for breast and gynecological cancers on lymphedema progression.
Conclusions
RT is an integral tool for treating certain cancers, as it extends disease-free survival, overall survival and reduces regional failure. However, RT increases the risk of lymphedema through multiple avenues as discussed specifically for breast and gynecological cancers. Decreasing the proliferative potential of lymphatic tissue, mechanical insufficiency of lymphatic vessels and lymph node fibrosis are some of the many ways RT leads to lymphedema. It is thus integral to understand the effect radiation has on the lymphatic vessels and glands, in order to individually weigh the costs versus the benefits and better inform patients clinically.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Xiaona Lu, Antonio Jorge Forte) for the series “Lymphedema” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs.2020.03.20). The series “Lymphedema” was commissioned by the editorial office without any funding or sponsorship. XL serves as the unpaid editorial board member of Gland Surgery from Aug 2019 to Jul 2021 and served as the unpaid Guest Editor of the series. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meek AG. Breast radiotherapy and lymphedema. Cancer 1998;83:2788-97. [Crossref] [PubMed]
- Hinrichs CS, Watroba NL, Rezaishiraz H, et al. Lymphedema secondary to postmastectomy radiation: incidence and risk factors. Ann Surg Oncol 2004;11:573-80. [Crossref] [PubMed]
- Erickson VS, Pearson ML, Ganz PA, et al. Arm edema in breast cancer patients. J Natl Cancer Inst 2001;93:96-111. [Crossref] [PubMed]
- Brennan MJ. Lymphedema following the surgical treatment of breast cancer: a review of pathophysiology and treatment. J Pain Symptom Manage 1992;7:110-6. [Crossref] [PubMed]
- Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg 1999;177:184-7; discussion 188. [Crossref] [PubMed]
- Moore KL, Persaud TVN, Torchia MG. The Developing Human-E-Book: Clinically Oriented Embryology: Elsevier Health Sciences, 2018.
- Yoffey JM, Courtice FC. Lymphatics, lymph and the lymphomyeloid complex. 1970.
- Mortimer PS. The pathophysiology of lymphedema. Cancer 1998;83:2798-802. [Crossref] [PubMed]
- Kubik S, Manestar M. The Initial Lymphatics 1985.
- Margaris KN, Black RA. Modelling the lymphatic system: challenges and opportunities. J R Soc Interface 2012;9:601-12. [Crossref] [PubMed]
- Smith RO. Lymphatic contractility: a possible intrinsic mechanism of lymphatic vessels for the transport of lymph. J Exp Med 1949;90:497-509. [Crossref] [PubMed]
- Lockwood-Rayermann S. Lymphedema in gynecologic cancer survivors: an area for exploration? Cancer Nurs 2007;30:E11-8. [Crossref] [PubMed]
- Lenzi M, Bassani G. The effect of radiation on the lymph and on the lymph vessels. Radiology 1963;80:814-7. [Crossref] [PubMed]
- Ariel IM, Resnick MI, Oropeza R. The effects of irradiation (external and internal) on lymphatic dynamics. Am J Roentgenol Radium Ther Nucl Med 1967;99:404-14. [Crossref] [PubMed]
- Fajardo LF. Effects of ionizing radiation on lymph nodes. In: The Lymphatic System and Cancer. Karger Publishers, 1994:37-45.
- Schünemann H, Willich N. Lymphoedema of the arm after treatment of cancer of the breast. A study of 5868 cases. Dtsch Med Wochenschr 1997;122:536-41. [PubMed]
- Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997;337:956-62. [Crossref] [PubMed]
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemo-therapy. N Engl J Med 1997;337:949-55. [Crossref] [PubMed]
- Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med 1993;328:1587-91. [Crossref] [PubMed]
- Sakorafas GH, Peros G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol 2006;15:153-65. [Crossref] [PubMed]
- Johansen J, Overgaard J, Blichert-Toft M, et al. Treatment morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol 2000;39:349-54. [Crossref] [PubMed]
- Yeoh EK, Denham J, Davies S, et al. Primary breast cancer complications of axillary management. Acta Radiol Oncol 1986;25:105-8. [Crossref] [PubMed]
- Kissin MW, Della Rovere GQ, Easton D, et al. Risk of lymphoedema following the treatment of breast cancer. Br J Surg 1986;73:580-4. [Crossref] [PubMed]
- Kwan W, Jackson J, Weir LM, et al. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol 2002;20:4242-8. [Crossref] [PubMed]
- Perez CA, Brady L. Principles and practice of radiation oncology, 1-55. Philadelphia: 1987.
- Moffat FL Jr, Senofsky GM, Davis K, et al. Axillary node dissection for early breast cancer: some is good, but all is better. J Surg Oncol 1992;51:8-13. [Crossref] [PubMed]
- Larson D, Weinstein M, Goldberg I, et al. Edema of the arm as a function of the extent of axillary surgery in patients with stage I–II carcinoma of the breast treated with primary radiotherapy. Int J Radiat Oncol Biol Phys 1986;12:1575-82. [Crossref] [PubMed]
- Goffman TE, Laronga C, Wilson L, et al. Lymphedema of the arm and breast in irradiated breast cancer patients: risks in an era of dramatically changing axillary surgery. Breast J 2004;10:405-11. [Crossref] [PubMed]
- Hayes SB, Freedman GM, Li T, et al. Does axillary boost increase lymphedema compared with supraclavicular radiation alone after breast conservation? Int J Radiat Oncol Biol Phys 2008;72:1449-55. [Crossref] [PubMed]
- Gross JP, Sachdev S, Helenowski IB, et al. Radiation Therapy Field Design and Lymphedema Risk After Regional Nodal Irradiation for Breast Cancer. Int J Radiat Oncol Biol Phys 2018;102:71-8. [Crossref] [PubMed]
- Helyer LK, Varnic M, Le LW, et al. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J 2010;16:48-54. [Crossref] [PubMed]
- Huang J, Yu N, Wang X, et al. Incidence of lower limb lymphedema after vulvar cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e8722. [Crossref] [PubMed]
- Mitra D, Catalano PJ, Cimbak N, et al. The risk of lymphedema after postoperative radiation therapy in endometrial cancer. J Gynecol Oncol 2016;27:e4. [Crossref] [PubMed]
- Tada H, Teramukai S, Fukushima M, et al. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer 2009;9:47. [Crossref] [PubMed]
- Todo Y, Yamamoto R, Minobe S, et al. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol Oncol 2010;119:60-4. [Crossref] [PubMed]
- Soisson AP, Soper JT, Clarke-Pearson DL, et al. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol 1990;37:390-5. [Crossref] [PubMed]
- Kragt H, Bouma J, Aalders J. Anticoagulants and the formation of lymphocysts after pelvic lymphadenectomy in gynecologic and oncologic operations. Surg Gynecol Obstet 1986;162:361-4. [PubMed]
- Werngren-Elgstrom M, Lidman D. Lymphoedema of the lower extremities after surgery and radiotherapy for cancer of the cervix. Rehabil Oncol 1997;15:32. [Crossref]
- Chatani M, Nose T, Masaki N, et al. Adjuvant radiotherapy after radical hysterectomy of the cervical cancer. Strahlentherapie und Onkologie 1998;174:504-9. [Crossref] [PubMed]
- Logsdon MD, Eifel PJ. Low dose rate brachytherapy in the treatment of cer-vical carcinoma. Hematol Oncol Clin North Am 1999;13:577-84. [Crossref] [PubMed]
- Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys 2001;51:880-914. [Crossref] [PubMed]
- Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003;56:83-8. [Crossref] [PubMed]
- Chang WI, Kang HC, Wu HG, et al. Lower Extremity Lymphedema in Gynecologic Cancer Patients: Propensity Score Matching Analysis of External Beam Radiation versus Brachytherapy. Cancers 2019;11:1471. [Crossref] [PubMed]
- Yost KJ, Cheville AL, Al-Hilli MM, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol 2014;124:307-15. [Crossref] [PubMed]
- Kim JH, Choi JH, Ki EY, et al. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. Int J Gynecol Cancer 2012;22:686-91. [Crossref] [PubMed]
- Kumar VJ, Nin CY, Kuei LY, et al. Survival and disease relapse in surgical stage I endometrioid adenocarcinoma of the uterus after adjuvant vaginal vault brachytherapy. Int J Gynecol Cancer 2010;20:564-9. [Crossref] [PubMed]
- Atlan D, Touboul E, Deniaud-Alexandre E, et al. Operable Stages IB and II cervical carcinomas: a retrospective study comparing preoperative uterovaginal brachytherapy and postoperative radiotherapy. Int J Radiat Oncol Biol Phys 2002;54:780-93. [Crossref] [PubMed]
- Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med 2015;373:307-16. [Crossref] [PubMed]
- Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. Lancet 2000;355:528-33. [Crossref] [PubMed]
- Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665-73. [Crossref] [PubMed]
- Yao Y, Chu Y, Xu B, et al. Radiotherapy after surgery has significant survival benefits for patients with triple-negative breast cancer. Cancer Med 2019;8:554-63. [Crossref] [PubMed]
- Homesley HD, Bundy BN, Sedlis A, et al. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol 1986;68:733-40. [PubMed]
- Fujiwara K, Suzuki S, Yoden E, et al. Local radiation therapy for localized relapsed or refractory ovarian cancer patients with or without symptoms after chemotherapy. Int J Gynecol Cancer 2002;12:250-6. [Crossref] [PubMed]
- Rose PG. Chemoradiotherapy for cervical cancer. Eur J Cancer 2002;38:270-8. [Crossref] [PubMed]
- Ridner SH, Dietrich MS, Deng J, et al. Bioelectrical impedance for detecting upper limb lymphedema in nonlaboratory settings. Lymphat Res Biol 2009;7:11-5. [Crossref] [PubMed]
- Erdogan Iyigun Z, Selamoglu D, Alco G, et al. Bioelectrical impedance for detecting and monitoring lymphedema in patients with breast cancer. Preliminary results of the florence nightingale breast study group. Lymphat Res Biol 2015;13:40-5. [Crossref] [PubMed]
- Bundred NJ, Stockton C, Keeley V, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015;151:121-9. [Crossref] [PubMed]
- Moseley A, Piller N. Reliability of bioimpedance spectroscopy and tonometry after breast conserving cancer treatment. Lymphat Res Biol 2008;6:85-7. [Crossref] [PubMed]
- Bagheri S, Ohlin K, Olsson G, et al. Tissue tonometry before and after liposuction of arm lymphedema following breast cancer. Lymphat Res Biol 2005;3:66-80. [Crossref] [PubMed]
- Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology 2001;34:2-11. [PubMed]
- Smoot BJ, Wong JF, Dodd MJ. Comparison of diagnostic accuracy of clinical measures of breast cancer–related lymphedema: area under the curve. Arch Phys Med Rehabil 2011;92:603-10. [Crossref] [PubMed]
- Hayes S, Sipio TD, Rye S, et al. Prevalence and prognostic significance of secondary lymphedema following breast cancer. Lymphat Res Biol 2011;9:135-41. [Crossref] [PubMed]
- Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest 2005;23:76-83. [Crossref] [PubMed]
- Ridner SH, Montgomery L, Hepworth J, et al. Comparison of upper limb volume measurement techniques and arm symptoms between healthy volunteers and individuals with known lymphedema. Lymphology 2007;40:35-46. [PubMed]
- Tierney S, Aslam M, Rennie K, et al. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg 1996;12:412-7. [Crossref] [PubMed]
- Stanton AW, Northfield JW, Holroyd B, et al. Validation of an optoelectronic limb volumeter (Perometer®). Lymphology 1997;30:77-97. [PubMed]
- Specht MC, Miller CL, Russell TA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression? Breast Cancer Res Treat 2013;140:485-94. [Crossref] [PubMed]
- Deltombe T, Jamart J, Recloux S, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology 2007;40:26-34. [PubMed]