Propeller thoracodorsal artery perforator flap for breast reconstruction
Introduction
Our group first described the thoracodorsal artery perforator flap (TDAP) in 1995, in an attempt to minimize donor-site complications related to the latissimus dorsi muscle cutaneous flap (1). Since then, several studies have demonstrated that the use of muscle-sparing latissimus dorsiflap is feasible and ensures excellent objective and subjective aesthetic outcomes without contour defect (2-4). Like the conventional muscle cutaneous flap, TDAP requires intramuscular dissection of the arterial perforator, and thus, it is more complex and time-consuming, entailing an additional learning curve for the surgeon.
In order to simplify breast reconstruction using a TDAP flap, we have modified the surgical technique by rotating the flap 180 degrees over the pedicle, to the mastectomy area (propeller technique), which eliminates the need for intramuscular pedicle dissection. In this report, we describe our clinical experience with the propeller TDAP flap during breast reconstructions.
Patients and methods
We performed a retrospective analysis to examine the outcomes associated with 17 patients who underwent propeller-shaped TDAP flaps (without intramuscular pedicle dissection) for breast reconstruction from January 2009 to February 2013. The ages of the patients ranged from 38 to 57 years. In 7 cases, the TDAP flap was designed in a horizontally, and the rest were created in an oblique upward position, using a Hammond-banana design (Table 1, Figures 1,2,3). Flap length ranged from 25-38 cm and width 8-10 cm (Table 1). In 15 cases this cutaneous branch was observed as a true muscular perforator of the latissimus dorsi muscle (83%) and in 3 cases (17%) there was a direct cutaneous branch coming from the descending branch. Operative time ranged 90-100 minutes. The flaps covered 90-95% of the width of the back. All flaps were vascularized by the proximal perforator of the descending branch of the thoracodorsal artery. The follow-up period ranged from 4 to 48 months. We collected data prospectively regarding demographics along with peri-operative, and postoperative outcomes. Prior to surgery, 14 patients underwent some type of chemotherapy, while four of them had also received breast radiation. Two patients had a bilateral reconstruction, one was immediate (patient with adenomastectomy for siliconomes), while the other was delayed (patient with prior mastectomy and radiotherapy). The rest (n=14) underwent a unilateral reconstruction. Indications for TDAP flaps (per breast) were as follows: skin reconstructions in 12 cases, skin reconstruction and volume enhancement in 5 and reconstructive volume enhancement in 1 (Table 1). In all cases, the indications for TDAP flaps were equally appropriate for latissimus dorsi flaps. The results were evaluated based on the survival of the flap and the associated donor site morbidity.
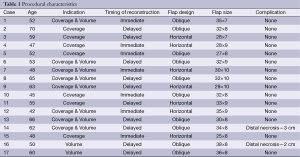
Full table
Technique
The flaps were designed with the patient in standing position, arms at the sides with the hands on the waist. Each patient was asked to actively contract her back muscles, with a cutaneous mark being made to represent the leading edge of the Latissimus dorsi muscle contraction. A point is marked on that line, 8 cm below the axillary fold. The descending branch of the proximal perforator artery runs parallel to that line, 2 cm lateral, approximately. The proximal perforator branch of the descending branch of the thoracodorsal artery pierces the muscle in the line of the descending branch at 8cm from the axillary fold or more. However, in 20% of the cases, a direct cutaneous branch from the descending branch of the thoracodorsal artery is the most important cutaneous branch (considering its diameter). This direct cutaneous branch does not pierce the muscle; it passes immediately anterior to the muscle lateral border. Thus, the design of the flap must exceed the edge of the muscle to assure the presence of this branch in the raised flap. The width of the flap is designed according to the possibility of direct closing of the donor site. The skin and the associated subcutaneous tissue are pinched with the thumb and index finger in order to mark the desired width. The flap’s length extends across the width of the back when the design is horizontal or across the superior inferior angle of the scapula when the design is made obliquely upward. We prefer the oblique, upward design because the thickness of the adipose tissue in the parascapular area provides more volume. The final choice, however, rests with each patient.
The location of the perforators is ideally determined using preoperative angiography and color Doppler ultrasonography. When these techniques are not available, the surgeon must rely on anatomical knowledge and clinical experience with the use of the flap, to locate these vessels that, in most cases, are in an area 8-cm below the axillary folds.
The flap is raised from distal to proximal direction, superficial to the deep fascia, while observing the fascia of the latissimus dorsi. The perforator arteries are carefully observed, using 4× magnifications, to detect any bleeding from the tip of the flap. The continuous and progressive control of the bleeding quality from the end portion of the flap represents an excellent way of monitoring the presence of a good perforator. If the flap has excellent perfusion by the time that it is half separated from the dorsal muscle, the perforator is likely to be adequate (diameter >0.5 mm). On the other hand, if a marked decrease in perfusion is detected when the flap is half raised and the intercostal perforators sectioned, we prefer to defer the procedure. Such a situation was not observed in this series. Dissection continues along the suprafascial plane to the anterior border of the muscle and it proceeds superiorly up to the perforator entrance point.
Locating the lateral edge of the muscle is important because the descending branch of the thoracodorsal artery runs parallel to that edge, at a distance of ≤2-4 cm. Therefore, the proximal perforator is found at approximately the same distance from the edge. In cases involving a direct cutaneous branch, this level is at the edge surrounding the muscle.
The proximal perforator artery also has an accompanying vein. Once this artery has been located, we perform complete dissection of the skin around the island itself. The dissection around the perforating artery is minimal, and serves to release the muscle and allow rotation of the flap along this axis, creating the “flap helix” (propeller) (5).
In cases of mastectomy sequelae, we release the scar and leave a gap to place the flap. In these cases, the previous scar incision is made in continuity with the flap incision. In the case of an immediate reconstruction, when performing skin sparing mastectomy or when no scar at the breast side is present, the flap is deepithelized and tunneled, remaining under the skin below the tunnel. Donor site closure is performed in two planes. A suction drain is placed and removed 48-72 hours after surgery.
Results
There were no donor-site seromas, and minimal wound dehiscence was detected in two cases. Minor skin paddle tip necrosis occurred in two flaps (34×8 and 38×8) which required tip resection. Both cases healed by second intention.
We found that tissular volume achievement with the TDAP flap is enough for symmetrization of the contralateral breast when facing a “A or B size cup”.
Case examples
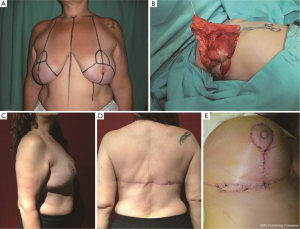
Case 1: a 48-year-old patient with multiple breast nodules and skin ulceration following 250 cc liquid silicone injection for breast augmentation in both breast (Figure 1A). We performed a staged bilateral adeno mastectomy with a Strombeck approach, utilizing two propeller TDAP flaps for breast reconstruction (Figure 1B,C). Note the final aesthetic results at 6 months (Figure 1D,E).
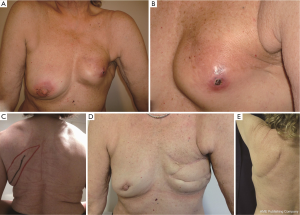
Case 2: a 68-year-old patient had immediate breast reconstruction with a 200 cc expander following simple mastectomy for the treatment of multicentric carcinoma. After the procedure, the patient received radiotherapy, which resulted into extrusion of the expander (Figure 2A,B). We removed the deflated expander and repaired the defect with a propeller TDAP flap with (Figure 2C,D) excellent results at two-month visit (Figure 2E).
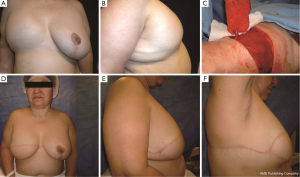
Case 3: a 53-year-old patient referred after suboptimal breast reconstruction with expander and ultimately prosthesis implantation. There was a deficit in the breast envelope (Figures 3 A,B). We decided to provide breast area coverage with a propeller TDAP flap (Figure 3C) without changing prosthesis volume. Note the aesthetic results at three months follow-up (Figure 3D,E).
Discussion
Over the past two decades, the ability for breast reconstruction has improved substantially. At first, musculocutaneous flaps (i.e., latissimus dorsi and TRAM, transverse rectus abdomino muscle cutaneous flap) remained the workhorse for coverage of most skin defects of the breast, but were progressively replaced by muscle-sparing flaps, owed to their lower morbidity at the donor-site and their greater precision during breast reconstruction (6-11). Specifically, the TDAP flap represents an extremely versatile muscle-sparing flap that possesses a reliable cutaneous blood supply arising from the lateral branch of the thoracodorsal artery (1,4). If needed, TDAP flaps can be rather large with a long pedicle length (up to 23 cm), which provides an extensive arc of rotation for pedicle transfers. Several reports have shown aesthetically pleasing donor site results following breast reconstruction with this flap-type, in part due to harvesting from a natural skin fold produced on lateral flexion without damaging the axillary silhouette (2,4). Consequently, donor site scars can usually be concealed underneath the arm or in the underwear. TDAP flaps have a thin subcutaneous fat tissue (commonly encountered at the back region), thus, providing a thin skin for precise breast reconstruction. Although there are other potentially useful muscle-sparing flaps with similar skin texture like the scapular and parascapular flaps, pedicles are shorter and hence, freedom is limited when compared to TDAP flaps.
The use of TDAP flaps is indicated for:
- Partial breast reconstructions;
- Combined with an expander or implant during complete breast reconstruction or;
- For further refinements when additional volume is required for reconstruction of the nipple-areola complex area.
Despite all advantages, harvesting TDAP flaps involves intramuscular dissection of the pedicle, a step that is particularly time-consuming. In an attempt to simplify this technique, we used TDAP propeller flaps, thus eliminating the need for intramuscular pedicle dissection.
According to the Tokyo Consensus, the term “propeller flap” describes a flap, based on a random subcutaneous pedicle, with a skin island of a length largely exceeding its width, made of two portions (the blades of the propeller), one at each side of the pedicle (12). Several authors have reported the application of the perforator propeller concept to the reconstruction of soft-tissue defects in different areas of the body (13-15).
In the current study, harvesting the TDAP propeller flaps was simple, feasible and safe. Donor-site aesthetic results were acceptable with minor complications and no long-term sequelae. Propeller flaps are expected to have the same complications as any other perforator flap. Venous congestion with necrosis at the flap tip, and distal flap tissue suffering for insufficient irrigation are the most frequent complication. This sign must not be interpreted as a venous congestion: it is produced by pressure decrease in the vascular system, slowing of blood flow and, thus, inappropriate capillary perfusion. Even though it looks like venous congestion, it can be solved by additional incorporation of arterial inflow and not by additional venous drainage. It is the distal limit of a possible flap designed with a determined pedicle. In this case, it is the distal limit of the TDAP.
Ischemia can be caused by an insufficient flow in the perforating vessel or by inadequate release of the fascial adhesions around the vascular pedicle and especially around the vein. In the present study, we identified minor necrosis at the tip of skin paddle in two excessively long flaps. Nowadays we refrain from harvesting flaps >30 cm in length.
Noteworthy, a propeller TDAP flap reach is shorter than conventional ones, as the pedicle length is restricted. Therefore, care should be taken when calculating the flap length. Although a propeller flap can usually be converted to a conventional perforator flap if needed, such surgical maneuver requires significant operator dexterity.
Conclusions
This preliminary study has demonstrated the feasibility and clinical versatility of the TDAP propeller flap in breast reconstruction. This flap is simple to harvest; it is associated with minimal donor site morbidity and emerges as optimal alternative during reconstructive breast surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg 1995;96:1608-14. [PubMed]
- Hamdi M, Salgarello M, Barone-Adesi L, et al. Use of the thoracodorsal artery perforator (TDAP) flap with implant in breast reconstruction. Ann Plast Surg 2008;61:143-6. [PubMed]
- Adler N, Seitz IA, Song DH. Pedicled thoracodorsal artery perforator flap in breast reconstruction: clinical experience. Eplasty 2009;9:e24. [PubMed]
- Yang JD, Kim MC, Lee JW, et al. Usefulness of Oncoplastic Volume Replacement Techniques after Breast Conserving Surgery in Small to Moderate-sized Breasts. Arch Plast Surg 2012;39:489-96. [PubMed]
- Thomsen JB, Bille C, Wamberg P, et al. Propeller TAP flap: is it usable for breast reconstruction? J Plast Surg Hand Surg 2013;47:379-82. [PubMed]
- Agaoglu G, Erol OO. Delayed breast reconstruction with latissimus dorsi flap. Aesthetic Plast Surg 2009;33:413-20. [PubMed]
- Durkin AJ, Pierpont YN, Patel S, et al. An algorithmic approach to breast reconstruction using latissimus dorsi myocutaneous flaps. Plast Reconstr Surg 2010;125:1318-27. [PubMed]
- Venus MR, Prinsloo DJ. Immediate breast reconstruction with latissimus dorsi flap and implant: audit of outcomes and patient satisfaction survey. J Plast Reconstr Aesthet Surg 2010;63:101-5. [PubMed]
- Bailey S, Saint-Cyr M, Zhang K, Mojallal A, et al. Breast reconstruction with the latissimus dorsi flap: women’s preference for scar location. Plast Reconstr Surg 2010;126:358-65. [PubMed]
- Drucker-Zertuche M, Robles-Vidal C. A 7 year experience with immediate breast reconstruction after skin sparing mastectomy for cancer. Eur J Surg Oncol 2007;33:140-6. [PubMed]
- Knight MA, Nguyen DT 4th, Kobayashi MR, et al. Institutional review of free TRAM flap breast reconstruction. Ann Plast Surg 2006;56:593-8. [PubMed]
- Pignatti M, Ogawa R, Hallock GG, et al. The “Tokyo” consensus on propeller flaps. Plast Reconstr Surg 2011;127:716-22. [PubMed]
- Sharma M, Balasubramanian D, Thankappan K, et al. Propeller flaps in the closure of free fibula flap donor site skin defects. Ann Plast Surg 2013;71:76-9. [PubMed]
- Schmidt VJ, Horch RE, Dragu A, et al. Myocutaneous propeller flap based on the superior gluteal artery (SGA) for closure of large lumbosacral meningomyelocoele defects: a case report. J Plast Reconstr Aesthet Surg 2012;65:521-4. [PubMed]
- Rad AN, Singh NK, Rosson GD. Peroneal artery perforator-based propeller flap reconstruction of the lateral distal lower extremity after tumor extirpation: case report and literature review. Microsurgery 2008;28:663-70. [PubMed]


