
The efficacy and safety of controlled low central venous pressure for liver resection: a systematic review and meta-analysis
Introduction
Hepatectomy is an effective method for the treatment of benign and malignant liver diseases. With recent improvements in surgical treatment, hepatectomy has been validated as an effective and safe surgical procedure and is widely recommended (1,2). Although preoperative strict selection criteria in patients and recent progress in surgical techniques and perioperative management have reduced the morbidity and mortality after hepatectomy, intraoperative bleeding is one of the major factors affecting the outcome of hepatectomy (3). A large number of studies have shown that operative mortality is related to intraoperative blood loss, and large blood loss can increase the incidences of postoperative complications and mortality (4).
Currently, the most commonly used method of hepatic blood flow occlusion in clinical practice is the Pringle method, but this method has a great impact on liver function and can cause hepatic ischemia-reperfusion injury (5). In addition, after blocking the blood flow into the liver, the main source of bleeding is the hepatic vein, how to control the hepatic vein bleeding is also the key to the operation. Therefore, the use of timely and effective bleeding control is of great clinical significance and practical value for rapid postoperative recovery and competent immune function in patients with benign and malignant liver diseases undergoing hepatectomy. Previous studies have shown that blood loss volume during hepatectomy is related to central venous pressure (CVP) (6). Intraoperative control of low central venous pressure (LCVP) is increasingly popular in hepatectomy, but its effectiveness and safety remain controversial. This study aimed to assess the role of controlled LCVP during hepatectomy and undertook the purpose of providing objective evidence for clinical decision-making.
Methods
Literature search strategy
As of December 2015, computer searches were performed on PubMed, EMBASE, Cochrane Library, and Google Scholar. The search words used were “central venous pressure” and “hepatectomy” or “hepatic dissection” or “liver resection”. In addition, we conducted a comprehensive manual search of the bibliography for each peer-reviewed paper selected. No language or date restrictions were imposed. Furthermore, there are no restrictions on the form of publication.
Study selection
All articles were independently reviewed by two authors (Wang and Sun). Any differences in whether a study should be chosen to be resolved by consensus. Inclusion criteria were established at the beginning of the study and were as follows: (I) population: patients with hepatectomy; (II) intervention: any method of LCVP; (III) results: operation time, blood loss, blood infusion, fluid infusion, urinary volume, alanine transaminase (ALT), total bilirubin (TBIL), blood urea nitrogen (BUN), creatinine (CR), postoperative complication rates and hospital stay; Methods: randomized controlled trials.
Data extraction
The two authors (Wang and Sun) conducted data extraction for each study. Any disagreement in data extraction is resolved by consensus. Detailed data, population, intervention, and outcome were recorded, including sample size, surgical procedure, LCVP control, operation time, blood loss, blood infusion, fluid infusion, urinary volume, ALT, TBIL, BUN, CR, postoperative complication rates and hospital stay.
Statistical analysis
The main result of the analysis was to reduce blood loss and blood infusion. Secondary outcomes included operative time, fluid infusion, urine volume, ALT, TBIL, BUN, CR, postoperative complication rates and length of hospital stay. Statistical analysis was employed using RevMan 5.3 software (Cochrane Collaboration, Oxford, England). The results of all studies were measured by mean ± standard deviation. If there is significant heterogeneity between the results (P<0.05), a random-effects model is used. A fixed-effect model was performed when there was no significant heterogeneity (P>0.05). Heterogeneity was evaluated using the Cochrane χ2 text (using a 10% significance level) and I2 statistic (the higher the percentage change in heterogeneity indicates a greater degree of heterogeneity). The OR of the result is 95% CI.
Results
Quality assessments
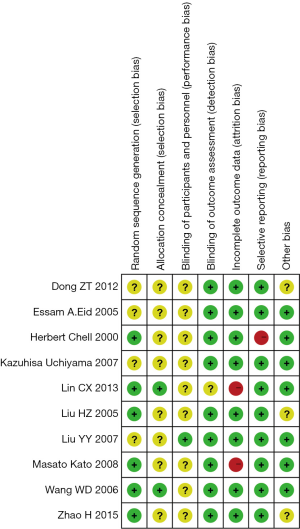
We generated a risk of bias graphs to identify the risk of bias of all included studies. The graphs showed that the studies included in our meta-analysis presented generally good methodological quality. The section bias of all studies experienced low risk especially the generation of random sequences. The unclear risk of bias was mainly focused on performance bias (blinding of participants and personnel). In a word, the included studies generally experienced good quality. The results of the risk of bias assessment are shown in Figure 1.
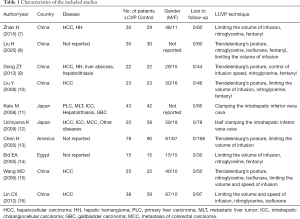
Characteristics of included studies
We identified 205 studies through an electronic search. Ten studies fulfilled the inclusion criteria of the meta-analysis (Figure 2). The characteristics of the 10 selected studies are shown in Table 1. Ten studies involved subjects, including 324 patients who underwent LCVP, and 393 who served as controls. The methods of controlled LCVP included Trendelenburg’s posture, nitroglycerine, furosemide, fentanyl, control of infusion speed, and clamping the intrahepatic vena cava (IVC) (17).
Full table
Outcome measures
Operation time
Six studies reported the data for the operative time and the difference between the LCVP and Control groups was not significant (MD: –16.24; 95% CI: –39.56 to 7.09; P=0.17; Figure 3A), with marked heterogeneity (P=0.005; I2=70%).
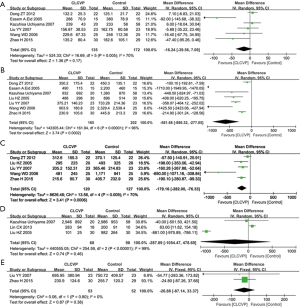
Blood loss
The meta-analysis of seven studies indicated that the volume of intraoperative blood loss in LCVP group was notably lower than that in Control group (MD: –581.68; 95% CI: –886.32 to –277.05; P=0.0002; Figure 3B), with marked heterogeneity (P<0.00001; I2=96%).
Blood infusion
Five trials reported information on blood infusion. The meta-analysis of the studies on blood infusion showed that blood infusion (MD: –179.16; 95% CI: –282.00 to –76.33; P=0.0006; Figure 3C) in LCVP group were significantly lower than in Control group (P=0.009; I2=70%).
Fluid infusion
Three studies reported the data for the fluid infusion and the difference between the LCVP and Control groups was not significant (MD: –287.89; 95% CI: –1,054.47 to 478.69; P=0.46; Figure 3D), with marked heterogeneity (P<0.00001; I2=99%).
Urinary volume
Two studies reported the data for the urinary volume and there was almost no difference between the LCVP and Control groups (MD: –26.88; 95% CI: –87.14 to 33.37; P=0.38; Figure 3E), with no heterogeneity (P=0.80; I2=0%).
ALT and TBIL
Three trials reported information on ALT, and two trials reported information on TBIL. Three studies reported the data for the ALT and the difference between the LCVP and Control groups was not significant (MD: –58.66; 95% CI: –153.81 to 36.50; P=0.23; Figure 4A), with marked heterogeneity (P=0.05; I2=67%). TBIL did not show a statistically significant difference between LCVP and Control groups (MD: –0.32; 95% CI: –3.93 to 3.28; P=0.86; Figure 4B), with no heterogeneity (P=0.61; I2=0%).
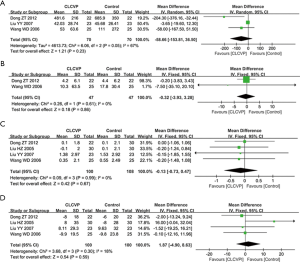
BUN and CR
Four trials were each reported information about BUN and CR. Four studies reported the data for the BUN and the difference between the CLCVP and Control groups was not significant (MD: –0.13; 95% CI: –0.73 to 0.47; P=0.67; Figure 4C), with no heterogeneity (P=0.99; I2=0%). CR also showed no statistically significant difference between LCVP and control group (MD: 1.87; 95% CI: –4.90 to 8.63; P=0.59; Figure 4D) with no heterogeneity (P=0.30; I2=18%).
Postoperative complication rates
Five studies reported the data for the postoperative complication rates and there was no difference between the LCVP and Control groups (MD: 0.62; 95% CI: 0.44 to 0.90; P=0.01; Figure 5A), with no heterogeneity (P=0.87; I2=0%).

Hospital stay
Two studies reported the data for the hospital stay and there was no difference between the LCVP and Control groups (MD: –0.61; 95% CI: –1.68 to 0.46; P=0.26; Figure 5B), with no heterogeneity (P=0.66; I2=0%).
Discussion
The incidence of liver disease is getting higher and higher, and surgical resection is the preferred method of treatment (18). Because the liver itself has a rich blood supply, hepatectomy has often led to massive bleeding during surgery. Clinical studies have shown (19) that the average blood loss in patients undergoing hepatectomy is 700 mL, and 30% of patients require an intraoperative blood transfusion to maintain Blood perfusion of vital organs of the body. There are various complications and increased risk of malignant tumor metastasis during intraoperative and postoperative transfusion (20). Therefore, how to improve the safety and feasibility of hepatectomy has always been a problem. With the development of anesthesia techniques and concepts, lowering CVP can reduce the pressure in the hepatic vein and hepatic sinus and reduce the bleeding when the liver parenchyma is broken. This is the theoretical basis for the control of LCVP to reduce hepatic resection and hemorrhage (21). In this meta-analysis, when liver resection was performed in Trendelenburg’s position that is an effective technical aspect of LCVP, the drug was treated by maintaining the CVP below 5 mmHg, the infusion rate was controlled, and the IVC under the liver was clamped. We analyzed the efficacy and safety of LCVP by blood loss, blood infusion, fluid infusion, operation time, urinary volume, ALT, TBIL, BUN, CR, postoperative complication rates and hospital stay.
First of all, this study presented that blood loss and blood infusion in the LCVP group were significantly lower than the control group. A large number of studies have reported (22,23), LCVP technique is used for hepatectomy and liver transplantation, which can reduce hepatic venous pressure, thus effectively reducing intraoperative blood loss and blood transfusion, and reducing surgical risk. According to the Poiser leaf laminar flow formula, the amount of hemorrhage caused by hepatic vein injury is proportional to the pressure difference of the vessel wall and the fourth power of the vessel radius. When the CVP decreases, the inferior vena cava pressure follows. When CVP decreases, the pressure of inferior vena cava, hepatic vein and hepatic sinus also decrease, which reduces the pressure difference of blood vessel wall and the radius of the blood vessel, and thus significantly reduces the intraoperative blood loss of patients (24). However, some studies (25,26) have reported that CVP has nothing to do with blood loss from hepatectomy, and intraoperative blood loss is not significantly reduced in patients with relatively low CVP. The differences between these studies may be due to differences in patient populations. And the bleeding tendency of patients with benign or malignant liver damage may be different.
Moreover, this meta-analysis showed that there was no difference in fluid infusion between the LCVP group and the control group. We found that in one study (27), patients in the LCVP and control group had a fluid infusion of 283 mL and 200 mL. However, another study (23) reported fluid infusion of 101 and 982 mL in the LCVP and control group. Hence, fluid infusion varied widely in different studies. Further large RCTs are needed.
In addition, regarding the operation time, this meta-analysis showed no significant difference between the low CVP group and the control group. There are many factors that affect the operation time, such as intraoperative bleeding, the difficulty of surgery, and so on. It is understandable that less blood loss is favorable for the surgical visual field and procedure, and reduces the duration of operation, reducing the operation time. However, difficult surgery usually takes longer, resulting in more blood loss, blood transfusion, and higher blood transfusion rates. Therefore, LCVP is necessary for difficult operations. For uncomplicated surgery, especially experienced surgeons, LCVP may not be needed.
Although the use of LCVP in hepatectomy has become increasingly popular, its widespread use is still limited. There is concern about the possibility that the lack of abdominal organ perfusion during LCVP during liver resection may lead to morbidity (28). In particular, the effects of relative hypotension and potential hypoperfusion on liver and kidney function have been listed as limiting factors. Our results showed no significant difference in ALT, TBIL, BUN, CR, and urine volume between the two groups. The results revealed that all tissues and organs were still effectively performed without affecting liver and kidney function in the case of LCVP. There is no significant effect on systemic hemodynamics, which is safe and reliable. The above results of our analysis are consistent with many reported studies (10,15,28).
Regarding the incidences of postoperative complications and postoperative hospital stay, this meta-analysis showed no significant difference between the low CVP group and the control group. This is also consistent with many studies (15,17). We hypothesize that the use of LCVP in hepatectomy may result in changes in cardiac preload and insufficient capacity, which may, in turn, affect the perfusion of vital organs, which may offset the benefits of low CVP and low blood loss surgery. However, some studies (27,29) pointed out that LCVP is performed during hepatectomy, and the reperfusion is guided by cardiac stroke volume variation. Compared with conventional infusion, less infusion volume is safe for patients and can reduce the incidence of postoperative complications. We believe that how to choose a better control of LCVP has a certain significance in the reduction of postoperative complication rate and the shortening of postoperative hospital stay.
This meta-analysis also has some limitations. First, one limitation of this meta-analysis is that the controls in each eligible study are various, and the number of tests is insufficient; Second, heterogeneity exists between trials, and there are many factors contributing to heterogeneity, such as differences in LCVP methods. Third, another limitation of this study is that it is based on retrospective studies with inherently unavoidable selection bias. Hence, further large randomized controlled trials are needed.
Conclusions
The present systematic review and meta-analysis found that the LCVP technique is safe and effective in hepatectomy. It has further clinical research and application value.
Acknowledgments
Funding: This work was supported by grants from the National Natural Scientific Funding (H1617); the project “medical leading talent and innovation team” (LJ201134) of Jiangsu Province.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs.2020.03.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dutta R, Mahato RI. Recent advances in hepatocellular carcinoma therapy. Pharmacol Ther 2017;173:106-17. [Crossref] [PubMed]
- Guo T, Ding R, Yang J, et al. Evaluation of different antibiotic prophylaxis strategies for hepatectomy: A network meta-analysis. Medicine (Baltimore) 2019;98:e16241. [Crossref] [PubMed]
- Moggia E, Rouse B, Simillis C, et al. Methods to decrease blood loss during liver resection: a network meta-analysis. Cochrane Database Syst Rev 2016;10:CD010683. [PubMed]
- Zerillo J, Agarwal P, Poeran J, et al. Perioperative Management in Hepatic Resections: Comparative Effectiveness of Neuraxial Anesthesia and Disparity of Care Patterns. Anesth Analg 2018;127:855-63. [Crossref] [PubMed]
- Lim C, Osseis M, Lahat E, et al. Extracorporeal Pringle Maneuver during Laparoscopic and Robotic Hepatectomy: Detailed Technique and First Comparison with Intracorporeal Maneuver. J Am Coll Surg 2018;226:e19-25. [Crossref] [PubMed]
- Ryckx A, Christiaens C, Clarysse M, et al. Central Venous Pressure Drop After Hypovolemic Phlebotomy is a Strong Independent Predictor of Intraoperative Blood Loss During Liver Resection. Ann Surg Oncol 2017;24:1367-75. [Crossref] [PubMed]
- Zhao H, Wang Y, Zhang X, et al. Application of controlled low central venous pressure combined with hepatic blood occlusion in hepatectomy. Chin J Clin Oncol 2015.1174-7.
- Liu H, Zhou Q, Wang X. Application of low central venous pressure in liver resection. Chin J Hepatobil Surg 2005;11:461-3.
- Dong ZT, Luo KL, Wu GZ, et al. Application of selective semi-hepatic vascular occlusion combined with low central venous pressure in hepatectomy. World Chinese J Digestol 2013;21:541. [Crossref]
- Liu Y, Cai M, Duan S, et al. Effect of controlled low central venous pressure on renal function in major liver resection. The Chinese-german J Clin Oncol 2008;7:7-9. [Crossref]
- Kato M, Kubota K, Kita J, et al. Effect of infra-hepatic inferior vena cava clamping on bleeding during hepatic dissection: a prospective, randomized, controlled study. World J Surg 2008;32:1082-7. [Crossref] [PubMed]
- Uchiyama K, Ueno M, Ozawa S, et al. Half clamping of the infrahepatic inferior vena cava reduces bleeding during a hepatectomy by decreasing the central venous pressure. Langenbecks Arch Surg 2009;394:243-7. [Crossref] [PubMed]
- Chen H, Merchant N, Didolkar MS. Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anesthesia improves morbidity and mortality. J Gastrointest Surg 2000;4:162-7. [Crossref] [PubMed]
- Eid EA, Sheta SA, Mansour E. Low central venous pressure anesthesia in major hepatic resection. Middle East J Anaesthesiol 2005;18:367. [PubMed]
- Wang WD, Liang LJ, Huang XQ, et al. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol 2006;12:935-9. [Crossref] [PubMed]
- Lin CX, Guo Y, Lau WY, et al. Optimal central venous pressure during partial hepatectomy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2013;12:520-4. [Crossref] [PubMed]
- Li Z, Sun Y, Wu F, et al. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J Gastroenterol 2014;20:303-9. [Crossref] [PubMed]
- Van Mierlo KM, Schaap FG, Dejong CHC, et al. Liver resection for cancer: New developments in prediction, prevention and management of postresectional liver failure. J Hepatol 2016;65:1217-31. [Crossref] [PubMed]
- Chen YJ, Zhen ZJ, Chen HW, et al. Laparoscopic liver resection under hemihepatic vascular inflow occlusion using the lowering of hilar plate approach. Hepatobiliary Pancreat Dis Int 2014;13:508-12. [Crossref] [PubMed]
- Postlewait LM, Squires MH, Kooby DA, et al. The relationship of blood transfusion with peri-operative and long-term outcomes after major hepatectomy for metastatic colorectal cancer: a multi-institutional study of 456 patients. HPB 2016;18:192-9. [Crossref] [PubMed]
- Otsubo T. Control of the inflow and outflow system during liver resection. J Hepatobiliary Pancreat Sci 2012;19:15-8. [Crossref] [PubMed]
- Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid 2010;20:135-46. [Crossref] [PubMed]
- Smyrniotis V, Kostopanagiotou G, Theodoraki K, et al. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg 2004;187:398-402. [Crossref] [PubMed]
- Vassiliou I, Arkadopoulos N, Stafyla V, et al. The introduction of a simple maneuver to reduce the risk of postoperative bleeding after major hepatectomies. J Hepatobiliary Pancreat Surg 2009;16:552-6. [Crossref] [PubMed]
- Niemann CU, Feiner J, Behrends M, et al. Central venous pressure monitoring during living right donor hepatectomy. Liver Transpl 2007;13:266-71. [Crossref] [PubMed]
- Chhibber A, Dziak J, Kolano J, et al. Anesthesia care for adult live donor hepatectomy: Our experiences with 100 cases. Liver Transpl 2007;13:537-42. [Crossref] [PubMed]
- Wang B, He H, Cheng B, et al. Effect of low central venous pressure on postoperative pulmonary complications in patients undergoing liver transplantation. Surg Today 2013;43:777-81. [Crossref] [PubMed]
- Correa-Gallego C, Berman A, Denis SC, et al. Renal function after low central venous pressure-assisted liver resection: assessment of 2116 cases. HPB 2015;17:258-64. [Crossref] [PubMed]
- Correa-Gallego C, Tan KS, Arslan-Carlon V, et al. Goal-Directed Fluid Therapy Using Stroke Volume Variation for Resuscitation after Low Central Venous Pressure-Assisted Liver Resection: A Randomized Clinical Trial. J Am Coll Surg 2015;221:591-601. [Crossref] [PubMed]


